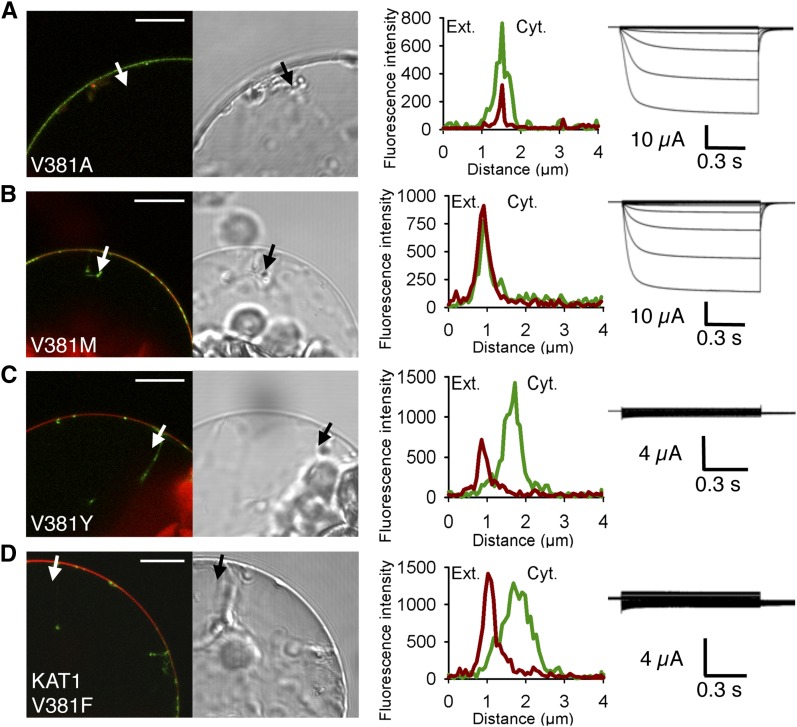
Figure 4.
Substitution of the C-linker Val residue by the aromatic amino acid Tyr in KAT2 and another Shaker channel from Arabidopsis, KAT1, results in impaired channel targeting to the PM. A to C, Substitution of Val-381 by Ala (A), Met (B), or Tyr (C) in KAT2. D, Substitution of Val-381 by Tyr in KAT1. Surface expression and activity at the PM of the mutated channels were investigated by subcellular localization of GFP fusions in tobacco protoplasts and current recordings in X. laevis oocytes, as described in the legend to Figure 1. A white arrow on the confocal image marks the position of the analyzed section crossing the PM and pockets of cytoplasm. Bars = 10 μm.