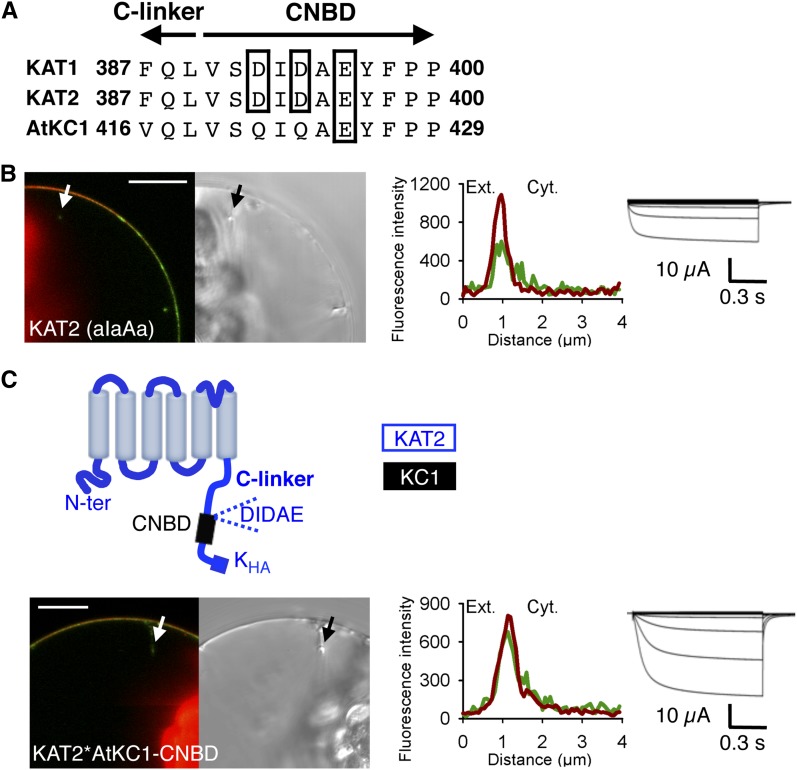
Figure 5.
KAT2 surface expression is not abolished by deletion of its triacidic motif DIDAE or substitution of its CNBD by that of AtKC1. A, Alignment of KAT1, KAT2, and AtKC1 sequences at the junction between the C-linker and the CNBD domains. Acidic amino acids of the triacidic motif DIDAE are boxed. Arrows indicate the C-linker and CNBD sequences. B, Mutation of the triacidic motif DIDAE into aIaAa in KAT2 does not prevent channel surface expression. C, Surface expression of the chimera obtained by replacing, in KAT2, the CNBD sequence downstream of the DIDAE motif by the corresponding sequence from AtKC1. A pictogram of the resulting chimera, displaying the sequences from KAT2 (blue) and AtKC1 (black), is provided at the top. Surface expression and activity at the PM of the KAT2(aIaAa) mutant (B) and the KAT2*AtKC1-CNBD chimera (C) were investigated by subcellular localization of GFP fusions in tobacco protoplasts and current recordings in X. laevis oocytes, as described in the legend to Figure 1. A white arrow on the confocal image marks the position of the analyzed section crossing the PM and pockets of cytoplasm. Bars = 10 μm.