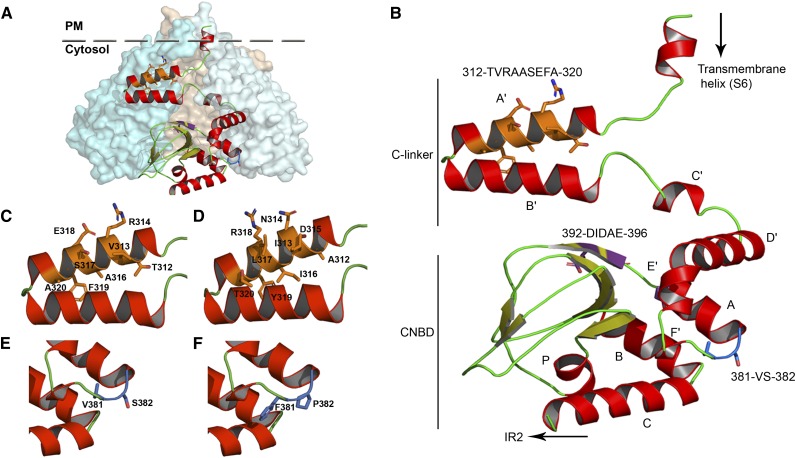
Figure 8.
Homology model of the C-linker and CNBD domains of KAT2. A, Assembly of the KAT2 C-linker and CNBD domains in a tetrameric configuration. One subunit is shown in ribbon display, while the other three are depicted in globular display, with each subunit colored differently. B, Ribbon representation of the KAT2 region (Met-295 to Gly-499) encompassing the end of the last transmembrane segment (S6), the C-linker domain (six α-helices [A′ to F′] separated by short loops), and the CNBD (one α-helix [A], a β-roll formed by β-strands, followed by two α-helices [B and C]). The α-helices are shown in red, and the β-strands are shown in green-yellow. The positions of the three sequences identified in this article (312TVRAASEFA320, 381VS382, and 392DIDAE396) are highlighted in orange, blue, and purple, respectively, and their corresponding side chains are displayed. C and D, Closeups of A′ and B′ α-helices of the KAT2 C-linker containing the native 312TVRAASEFA320 (C) and the mutated 312AINDILRYT320 (D) sequences. E and F, Closeups of the linker residues between F′ and A α-helices containing the native 381VS382 (E) and the mutated V381F/S382P (F) sequences.