Abstract
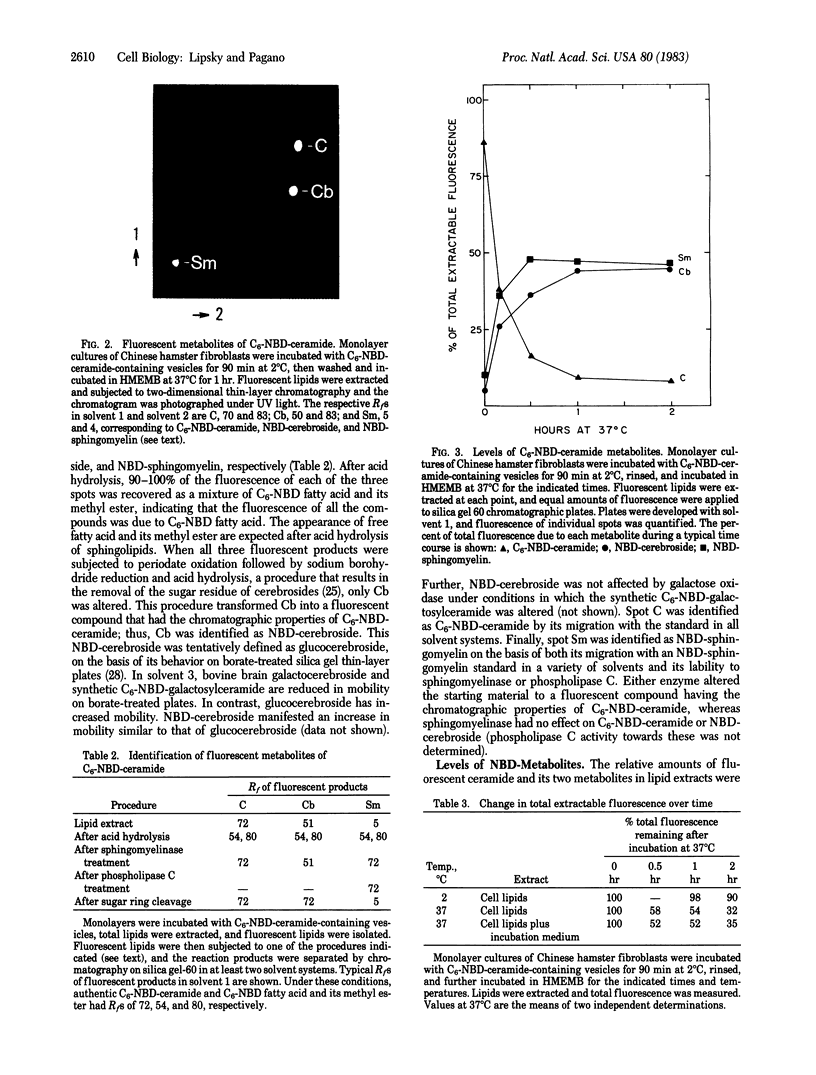
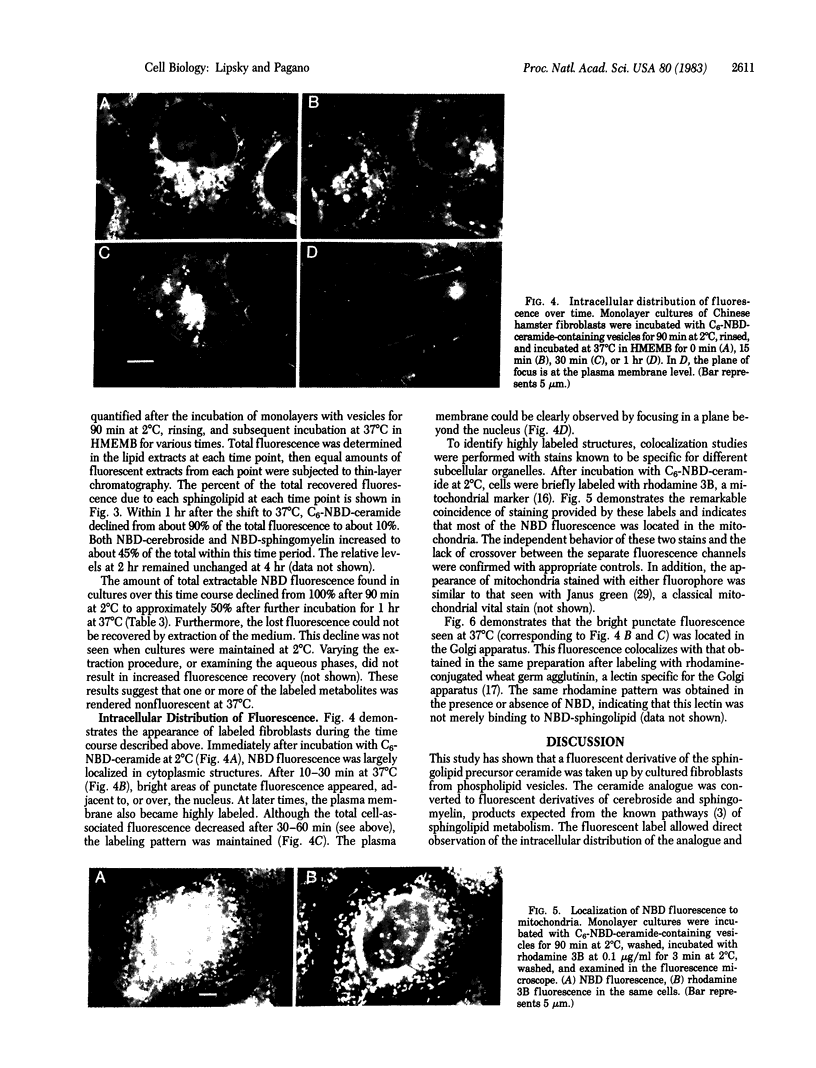
A fluorescent analogue of ceramide, N-[7-(4-nitrobenzo-2-oxa-1,3-diazole)]-epsilon-aminocaproyl sphingosine (C6-NBD-ceramide), was used to investigate sphingolipid metabolism in Chinese hamster fibroblasts. C6-NBD-ceramide was incorporated into small unilamellar dioleoyl phosphatidylcholine vesicles and incubated with cells in monolayer culture at 2 degrees C, resulting in rapid and preferential transfer of the labeled ceramide from vesicles to cells. The cells were then washed and subsequently incubated at 37 degrees C for various intervals. The metabolism of C6-NBD-ceramide was monitored by lipid extraction and analysis, and the intracellular distribution of the labeled molecule was followed by fluorescence microscopy. Initially, fluorescence was detected almost exclusively in mitochondria, with over 90% of the extractable lipid fluorescence due to C6-NBD-ceramide. After 30 min at 37 degrees C, intense fluorescence appeared in the Golgi apparatus. This organelle was identified by colocalization of NBD fluorescence with a Golgi-apparatus-specific stain. At later times the plasma membrane became visibly labeled as well, at which point 90% of the cell-associated fluorescence was recovered as NBD-labeled sphingomyelin and NBD-labeled cerebroside. These metabolites were identified by enzymatic and biochemical analysis and by thin-layer chromatography of the fluorescent lipid extracts. The finding that C6-NBD-ceramide is used by these cells in standard pathways of sphingolipid biosynthesis suggests that this fluorescent precursor will be a valuable tool for correlating the metabolism of sphingolipids with their intracellular distribution and translocation. In addition, during its metabolism by Chinese hamster fibroblasts, this compound acts as a vital stain for the Golgi apparatus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980 Sep 30;604(2):129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Bernert J. T., Jr, Ullman M. D. Biosynthesis of sphingomyelin from erythro-ceramides and phosphatidylcholine by a microsomal cholinephosphotransferase. Biochim Biophys Acta. 1981 Oct 23;666(1):99–109. doi: 10.1016/0005-2760(81)90095-3. [DOI] [PubMed] [Google Scholar]
- Brady R. O. Sphingolipidoses. Annu Rev Biochem. 1978;47:687–713. doi: 10.1146/annurev.bi.47.070178.003351. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. III. Reduction of Janus green B by isolated enzyme systems. Exp Cell Res. 1953 Sep;5(1):82–97. doi: 10.1016/0014-4827(53)90096-2. [DOI] [PubMed] [Google Scholar]
- Chen W. W., Moser A. B., Moser H. W. Role of lysosomal acid ceramidase in the metabolism of ceramide in human skin fibroblasts. Arch Biochem Biophys. 1981 May;208(2):444–455. doi: 10.1016/0003-9861(81)90531-2. [DOI] [PubMed] [Google Scholar]
- FORD D. K., YERGANIAN G. Observations on the chromosomes of Chinese hamster cells in tissue culture. J Natl Cancer Inst. 1958 Aug;21(2):393–425. [PubMed] [Google Scholar]
- Fambrough D. M., Devreotes P. N. Newly synthesized acetylcholine receptors are located in the Golgi apparatus. J Cell Biol. 1978 Jan;76(1):237–244. doi: 10.1083/jcb.76.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Zambrano F., Fleischer S. Biochemical characterization of the golgi complex of mammalian cells. J Supramol Struct. 1974;2(5-6):737–750. doi: 10.1002/jss.400020517. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J., Basu S. Ganglioside biosynthesis. Concentration of glycosphingolipid glycosyltransferases in Golgi apparatus from rat liver. J Biol Chem. 1974 Jan 10;249(1):310–315. [PubMed] [Google Scholar]
- Kishimoto Y., Kawamura N. Ceramide metabolism in brain. Mol Cell Biochem. 1979 Jan 15;23(1):17–25. doi: 10.1007/BF00226676. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Esker M. W., Pathmamanoharan C., Wiersema P. H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977 Aug 23;16(17):3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- Marggraf W. D., Anderer F. A., Kanfer J. N. The formation of sphingomyelin from phosphatidylcholine in plasma membrane preparations from mouse fibroblasts. Biochim Biophys Acta. 1981 Apr 23;664(1):61–73. doi: 10.1016/0005-2760(81)90028-x. [DOI] [PubMed] [Google Scholar]
- Miller-Podraza H., Fishman P. H. Translocation of newly synthesized gangliosides to the cell surface. Biochemistry. 1982 Jul 6;21(14):3265–3270. doi: 10.1021/bi00257a003. [DOI] [PubMed] [Google Scholar]
- Morell P., Braun P. Biosynthesis and metabolic degradation of sphingolipids not containing sialic acid. J Lipid Res. 1972 May;13(3):293–310. [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J., Martin O. C. Intracellular translocation and metabolism of a fluorescent phosphatidic acid analogue in cultured fibroblasts. J Biol Chem. 1983 Feb 10;258(3):2034–2040. [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J., Martin O. C., Struck D. K. Metabolism and intracellular localization of a fluorescently labeled intermediate in lipid biosynthesis within cultured fibroblasts. J Cell Biol. 1981 Dec;91(3 Pt 1):872–877. doi: 10.1083/jcb.91.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C., Schroit A. J., Struck D. K. Formation of asymmetric phospholipid membranes via spontaneous transfer of fluorescent lipid analogues between vesicle populations. Biochemistry. 1981 Aug 18;20(17):4920–4927. doi: 10.1021/bi00520a018. [DOI] [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Gal A. E., Hibbert S. R. The isolation and characterization of sphingomyelinase from human placental tissue. Biochim Biophys Acta. 1977 Aug 24;488(2):312–321. doi: 10.1016/0005-2760(77)90189-8. [DOI] [PubMed] [Google Scholar]
- Poulos A., Hann C., Phillipou G., Pollard A. C. The estimation of sphingolipids by gas chromatography-chemical ionization mass spectrometry of their derived aldehydes with particular reference to the ceramides of children's plasma. Anal Biochem. 1979 Sep 1;97(2):323–327. doi: 10.1016/0003-2697(79)90080-0. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Macpherson I. A. Glycolipid synthesis in normal and transformed animal cells. Proc R Soc Lond B Biol Sci. 1971 Feb 16;177(1046):49–58. doi: 10.1098/rspb.1971.0014. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Melzner I. Studies in vitro on the biosynthesis of ceramide and sphingomyelin. A reevaluation of proposed pathways. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):755–771. doi: 10.1515/bchm2.1980.361.1.755. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Pagano R. E. Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem. 1980 Jun 10;255(11):5404–5410. [PubMed] [Google Scholar]
- Tartakoff A. M. The Golgi complex: crossroads for vesicular traffic. Int Rev Exp Pathol. 1980;22:227–251. [PubMed] [Google Scholar]
- Ullman M. D., Radin N. S. The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J Biol Chem. 1974 Mar 10;249(5):1506–1512. [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker D. R., Kennedy E. P. Cellular and enzymic synthesis of sphingomyelin. Biochemistry. 1982 May 25;21(11):2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- Yedgar S., Gatt S. Enzymic hydrolysis of sphingomyelin in the presence of bile salts. Biochem J. 1980 Mar 1;185(3):749–754. doi: 10.1042/bj1850749. [DOI] [PMC free article] [PubMed] [Google Scholar]