Abstract
Background:
Diabetes and Tuberculosis often present together and complicate each other at many levels. A collaborative framework for care and control of diabetes and tuberculosis developed by World Health Organisation and International Union against Tuberculosis and Lung Diseases emphasizes routine bi-directional screening for the two diseases.
Aims:
The study was to assess the prevalence of diabetes in tuberculosis patients currently on treatment.
Materials and Methods:
This facility-based cross-sectional study was undertaken in four randomly selected peripheral health institutions providing directly observed treatment short-course, treatment for tuberculosis patients. All cases of tuberculosis, more than 18 years of age were screened for diabetes. Risk factors like age, sex, family history of diabetes, alcohol, smoking and obesity were assessed.
Results:
The prevalence of diabetes in tuberculosis patients was found to be 29% (known diabetics - 20.7%, new Diabetes cases - 8.3%). Diabetes was significantly associated with older age, family history of diabetes, consumption of alcohol and sputum positivity.
Conclusions:
Screening patients with Tuberculosis for fasting blood sugar estimation will help in early detection of diabetes
Keywords: Diabetes, directly observed treatment, short-course, therapy, prevalence, risk factors, tuberculosis
Introduction
About 95% of patients with tuberculosis (TB) and 70% of patients with diabetes mellitus (DM) live in the low and middle income countries.[1,2] The epidemic growth of DM has occurred in developing countries where TB is highly endemic. As a result, DM and TB are increasingly present together, and this calls for renewed interest in this topic.[3] India is facing the dual problem of being the highest TB-burden country having a large number of people with diabetes posing a serious challenge for the health system.[4,5]
Systematic reviews have shown scientific evidence of the linkages between the two diseases. Diabetes and tuberculosis may complicate each other at many levels. Among those with active TB, diabetes may adversely affect TB treatment outcomes by delaying the time for microbiological response, reducing the likelihood of favorable outcome and increasing the risk of relapse, death and drug resistance.[6,7]
The World Health Organization (WHO) and the International Union against Tuberculosis and Lung Disease (IUATLD) developed a collaborative framework for care and control of diabetes and tuberculosis, which emphasizes the routine implementation of bi-directional screening for the two diseases. It strongly recommends surveillance of diabetes among TB patients in all countries in primary healthcare settings. Screening for diabetes in patients with tuberculosis will not only ensure early case detection but also better management of diabetes. Tuberculosis patients accessing healthcare centers for directly observed treatment, short-course (DOTS) therapy for TB could also avail facilities for management of diabetes at the same time. This would thus reduce the cost incurred by both the patient and the health system and prevent unnecessary duplication of delivery of services. Co-located DOT centers and diabetic clinics at the primary health clinic (PHC) level will be a big leap forward in early detection and management of the two diseases. Moreover, the success of DOTS therapy for TB treatment can be used in management of diabetes, emphasizing standardized protocols, supervision of treatment, reliable supply of quality assured drugs, regular monitoring and evaluation and strong political will. Studies on diabetes in TB patients in India are scarce and have used different techniques and criteria for diagnosis of DM. This study aims to estimate the prevalence of DM and its risk factors among the adult patients with TB for DOTS in urban Puducherry, India.
Materials and Methods
This facility-based cross-sectional study was undertaken in four randomly selected peripheral health institutions (PHI) out of 14 urban PHIs providing DOTS therapy for TB patients in Puducherry. The 14 urban PHIs were listed and numbered in an alphabetical order and then four were selected randomly using random numbers from the random number table. All TB cases more than 18 years of age, including new and re-treatment cases, sputum positive, sputum negative and extra-pulmonary cases currently on treatment in the 4 selected PHIs were included in the study. The total number of patients currently on treatment in the 4 PHIs during the study period was found to be 223. All of these were screened for diabetes using the diagnostic criteria of a fasting plasma glucose level of ≥ 126 mg/dl[8] or a self-reported history of taking anti-diabetic drugs after diagnosis by a medical professional.
Firstly, a list of all TB patients currently on DOTS in that PHI was made. The days of DOTS therapy was ascertained for all patients listed. On the first day, the purpose of the study was explained to the patient and participant information sheet written in Tamil (local language) was provided and informed written consent was obtained. All the willing participants were interviewed using the pretested questionnaire. The questionnaire, based on the WHO-STEPS questionnaire, contained questions to assess risk factors of diabetes namely age, sex, family history of diabetes, alcohol and smoking. Physical parameters like height, weight, body mass index (BMI), waist circumference, hip circumference and waist-hip ratio were recorded.
In the patients who had already been diagnosed for diabetes, details of the time of diagnosis and treatment taken were collected. Family history of diabetes was enquired in all subjects. Self-reported information on tobacco usage and alcohol consumption was collected. Current usage of tobacco or alcohol; number of cigarettes smoked per day and number of years of smoking; average daily quantity and frequency of alcohol consumed were ascertained.
The height, weight, waist circumference and hip circumference were measured. The weight was taken on a weighing scale with standard minimum clothing to the nearest 0.5 kg. Height was measured on a vertical scale with the heel, buttocks and occiput against the wall and the head in the frankfurt plane to the nearest 0.5 cm. Waist circumference (WC) was measured at the level of the highest point of the iliac crest and hip circumference was the largest diameter around the buttocks. The cutoff for WC to quantify abdominal obesity indicating high risk was taken as 90 cm for men and 80 cm for women. Waist-hip ratio was calculated by using the formula WHR= (waist circumference in cm)/(Hip circumference in cm). WHR of more than 0.85 in women and 0.95 in man was considered as abdominal obesity. BMI was calculated by the formula BMI = (weight in kg)/(height in mts)2. BMI was used to categorize the nutritional status of the subjects as per the recommended cutoffs for Asian Indians. (Normal BMI: 18.0-22.9 kg/m2; Overweight: 23.0-24.9 kg/m2; Obesity: >25 kg/m2 ).[9]
The participants were asked to come the next day after overnight fasting. The fasting blood glucose levels of the patient were measured using a standardized glucometer available at the PHCs itself. Patients with abnormal fasting blood sugar values or presence of two or more risk factors were referred to the medical officer-in-charge of that PHI and treated accordingly.
Details from the tuberculosis card
Details about the category of treatment; i.e., category 1 or category 2 and sputum status at the time of diagnosis; i.e., sputum positive, sputum negative or extra-pulmonary TB, were noted from the TB treatment card.
All information obtained from the patients were kept confidential. The study was initiated after the approval from the Institute Ethical Committee. Participant information sheet in Tamil was provided to each patient and informed written consent was obtained. Permission from the health authorities was also taken prior to the study.
Statistical analysis
Data was entered in Microsoft excel 2007 and analyzed using Statistical Package for the Social Sciences software (SPSS v17.0). Continuous variables were summarized as mean with standard deviation (SD) and t-test was used to compare means. Categorical variables were expressed as counts (proportions) and Chisquare analysis were performed to compare proportions. A P < 0.05 was taken as statistically significant.
Results
Out of the 223 subjects enrolled for this study, complete details and fasting blood sugar values were available for 217 subjects of which 45 (20.7%) were females. The mean age for males and females was 44.9 ± 12.9 and 36.2 ± 16.2 years, respectively. About 66.3% of the females and 88.2% of the males were married and one-fourth of the subjects were illiterate. About 29% of the study population were unemployed, half of whom comprised of women who were homemakers. Occupation-wise, the largest proportion consisted of unskilled workers. [Table 1] gives the gender-wise distribution of certain characteristics of the study population.
Table 1.
Gender-wise distribution of certain characteristics of the study population
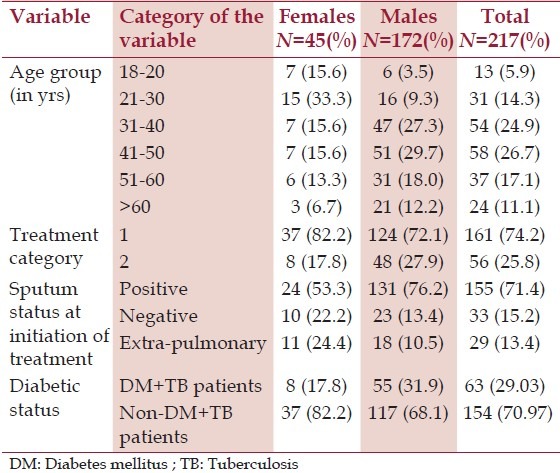
Using the diagnostic criteria, as mentioned in the methodology, the prevalence of diabetes among TB patients in this study was found to be 29% of which 20.7% were known DM cases and 8.3% were newly diagnosed. The prevalence was found to be more in males when compared to females. Impaired fasting glucose was seen in 15.1% of the TB patients. Among the known diabetics; diabetes antedated TB by a mean duration of 48.7 months.
On analyzing the risk factors of diabetes in TB patients, about 14% of the TB patients had a family history of diabetes. About two-thirds of males use tobacco in the form of cigarettes; i.e., smoking about 14.8 cigarettes in a day. The average duration of smoking among smokers is 15.1 ± 12.9 years. Similarly, two-thirds of males consume alcohol with an average daily consumption of 295 ± 75.9 ml per day. Majority of the TB patients were underweight with a mean weight of 46.8 ± 11.4 kg. The mean BMI was 17.8 ± 4.4 kg/m2 (females = 18.7 ± 5.3, males = 17.6 ± 4.2 kg/m2 ). Overweight and obesity was observed in 11.9% of the subjects; more in females when compared to males (15.6% vs 11.0%). The BMI of the TB patients with diabetes was higher when compared to the TB patients without diabetes (18.92 ± 4.83 vs 17.39 ± 4.23), but the difference was not statistically significant. Abdominal obesity as indicated by high waist circumference and high waist hip ratio was seen in 9.6% and 48.9% of the subjects respectively; being significantly more in females when compared to males (26.7% vs 5.2% and 80% vs 40.7, respectively). About 74.2% of the subjects were new cases under category 1 treatment and 71.4% were sputum positive at treatment initiation. Univariate analysis of the prevalence of diabetes and its risk factors in TB patients is shown in [Table 2]. Significant association was seen with respect to age, family history of diabetes and consumption of alcohol and sputum positivity at the initiation of treatment.
Table 2.
Univariate analysis of prevalence of diabetes and its risk factors in TB patients
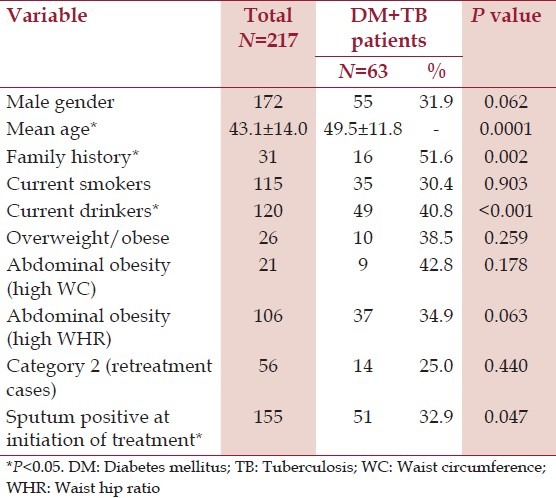
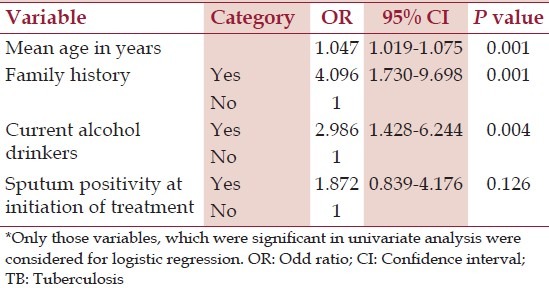
Variables found significant in the univariate analysis were included for binary logistic regression. Age, family history, and current alcohol consumption were found to be independent risk factors for diabetes in TB patients in the binary logistic regression analysis [Table 3]. After the age of 18, unit increase in age increased the odds of diabetes by 1.047 times. The odds of developing diabetes were 4.09 times among patients with a family history of diabetes and 2.98 times among the current alcohol consumers. In this study, sputum positivity at the initiation of treatment was not a significant risk factor for diabetes.
Table 3.
Logistic regression analysis of risk factors* for diabetes in TB patients
Discussion
In 200, a population-based study conducted in six large cities from different regions of India estimated an age-standardized prevalence of type 2 diabetes to be 12.1%.[10] Similarly, studies from urban Pondicherry estimated a prevalence of diabetes to be 5.6%, 8.27% and 8.6%, respectively with a prevalence of 5.8% in rural areas of Puducherry.[11,12,13,14] In the current study, the prevalence of diabetes in TB patients was found to be 29% (known DM cases - 20.7%, new DM cases - 8.3%). Thus, the prevalence of diabetes in TB patients in this study is much higher than the prevalence seen in the general population.
Similar prevalence was reported by studies from Saudi Arabia and Tamil nadu.[15,16] The study from Saudi Arabia by Singla et al., was a retrospective study done in inpatients during the year 1998-99. This inpatient cohort may not be representative of all TB patients being treated as TB patients requiring admission who usually have more severe disease. The study from Tamil Nadu estimated a diabetes prevalence of 25% among TB patients which was higher when compared to the prevalence of diabetes of 10% in the general population.[15] A study of higher prevalence of 44% was reported from Kerala, India. This study had used a different diagnostic criteria, i.e. measurement of HbA1c >6.5% to diagnose diabetes.[17] The WHO-IUALTD collaborative framework suggests that the type of screening and diagnostic tests for DM in TB patients should be adapted to the context of local health systems and the availability of resources.[6] Studies from China and Indonesia have demonstrated a lower prevalence.[18,19] Study by Jain et al. reported a prevalence of impaired glucose tolerance (IGT) of 16.98% and they had used oral glucose tolerance test to diagnose IGT.[20]
The present study has found a significantly higher prevalence of diabetes in older TB patients. Similar findings have been reported by studies from India[20] and other countries like Indonesia, Malaysia, Saudi Arabia, Taiwan and Mexico.[16,19,21,22] With the demographic transition underway globally, increase in life expectancy, improvements in provision of health services and a subsequent increase in the elderly population, the absolute numbers of cases of diabetes will increase exponentially. Routine screening of TB patients for DM will help detect cases of diabetes and pre-diabetes early, so that primary prevention methods may be initiated early and effectively. Government of India recommends that TB patients should be screened for DM immediately after the diagnosis of TB, but can also be done at anytime during the course of TB treatment.
This study has not found any significant association between BMI or waist circumference and diabetes. Similar results were reported by studies by Jain et al. A few studies have reported that patients with TB and DM are significantly underweight and have more weight loss.[22,23] Alisjahbana et al. have reported a significantly higher median BMI in TB-DM patients when compared to non-diabetic TB patients.[19] The sample size of this study may not be sufficient to demonstrate the presence, if any, of any underlying significant association.
This study has reported a significant association between alcohol consumption and prevalence of diabetes, which has not been stated elsewhere. This may be due to easy availability of alcohol in the area. Most of the studies have demonstrated a higher association of diabetes with sputum positivity.[16,19] Two studies have reported that DM antedated TB diagnosis by 4-5 years similar to the present study.[19,21]
This study has estimated the prevalence of DM in TB but further research is needed to ascertain, the best time for screening, identify a valid and cost-effective method of screening and subsequent changes needed, if any, in the standard management guidelines in patients with both diseases. There are numerous issues of basic, applied and operational research waiting for solutions. This converging of two epidemics should be a wake-up call for all clinicians and researchers to gear-up to meet the challenge of patients afflicted by tuberculosis as well as diabetes. It is time that the “unhealthy partnership” of tuberculosis and diabetes receives the attention it deserves. Being forewarned and prepared gives a better chance of reducing the dual burden of disease of DM and TB.[2,24]
Conclusion
Diabetes is a common co-morbidity in people with TB. Screening patients with TB with fasting blood sugar estimation will help in early detection of diabetes. Strategies are needed to ensure that optimal care is provided to patients with both diseases.
Acknowledgment
This study was undertaken as a short term studentship (STS) project of Indian Council of Medical Research (ICMR). The authors duly acknowledge the STS program of ICMR which supported the research.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Harries AD, Murray MB, Jeon CY, Ottmani SE, Lonnroth K, Barreto ML, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health. 2010;15:659–63. doi: 10.1111/j.1365-3156.2010.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen T, Joshi SR, Udwadia ZF. Tuberculosis and Diabetes Mellitus: Merging epidemics. J Assoc Physicians India. 2009;57:399–404. [PubMed] [Google Scholar]
- 3.Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, Crevel RV. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 4.TB India 2012: Annual Status Report, RNTCP. CTD, MOH&FW, New Delhi. 2012. [Accessed August 7, 2013]. at tbcindia.nic.in/pdfs/TB%20India%202012-%20Annual%20Report.pdf .
- 5.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 6.Collaborative framework for care and control of tuberculosis and diabetes: Report by WHO and IUATLD. Geneva: WHO; [Accessed September 15, 2013]. at whqlibdoc.who.int/publications/2011/9789241502252_eng.pdf . [PubMed] [Google Scholar]
- 7.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation. Geneva: WHO; [Accessed July 20, 2013]. at http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/index.html . [Google Scholar]
- 9.National Programme for Prevention and Control of Diabetes, Cardiovascular Disease and Stroke: A Manual for Medical Officer. Government of India – WHO Collaborative Programme. [Accessed July 10, 2013]. at www.searo.who.int/india/.../cardiovascular_diseases/NCD_Resources_Combined_ manual_for_medical_officers.pdf .
- 10.Mohan V, Pradeepa R. Epidemiology of diabetes in different regions of India. Health Administrator. 2009;22:1–18. [Google Scholar]
- 11.Purty AJ, Vedapriya DR, Bazroy J, Gupta S, Cherian J, Vishwanathan M. Prevalence of diagnosed diabetes in an urban area of Puducherry, India: Time for preventive action. Int J Diabetes Dev Ctries. 2009;29:6–11. doi: 10.4103/0973-3930.50708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SK, Singh Z, Purty AJ, Vishwanathan M. Diabetes prevalence and its risk factors in urban Puducherry. Int J Diabetes Dev Ctries. 2009;29:166–9. doi: 10.4103/0973-3930.57348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharati DR, Pal R, Kar S, Rekha R, Yamuna TV, Basu M. Prevalence and determinants of diabetes mellitus in Puducherry, South India. J Pharm Bioallied Sci. 2011;3:513–8. doi: 10.4103/0975-7406.90104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majgi SM, Soudarssanane BM, Roy G, Das AK. Risk factors of diabetes mellitus in rural Puducherry. Online J Health Allied Scs. 2012;11:4. [Google Scholar]
- 15.Screening of Tuberculosis Patients for Diabetes Mellitus: Pilot Project-,Module for RNTCP staff 2009. Central TB Division, MOH&FW, New Delhi [Google Scholar]
- 16.Singla R, Khan N, Al-Sharif N, Al-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–9. [PubMed] [Google Scholar]
- 17.Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Lin Y, Mi F, Tan S, Liang B, Guo C, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Trop Med Int Health. 2012;17:1294–301. doi: 10.1111/j.1365-3156.2012.03068.x. [DOI] [PubMed] [Google Scholar]
- 19.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 20.Jain MK, Baghel PK, Agrawal R. Study of impaired glucose tolerance in pulmonary tuberculosis. Indian J Community Med. 2006;31(3):137–9. [Google Scholar]
- 21.Chang JT, Dou HY, Yen CL, Wu YH, Huang RM, Lin HJ, et al. Effect of type 2 diabetes mellitus on clinical severity and treatment outcome in patients with pulmonary tuberculosis: A potential role in the emergence of multi-drug resistance. J Formos Med Assoc. 2011;110:372–81. doi: 10.1016/S0929-6646(11)60055-7. [DOI] [PubMed] [Google Scholar]
- 22.Guptan A, Shah A. Tuberculosis and Diabetes: An Appraisal. Ind J Tub. 2000;47(3):2–8. [Google Scholar]
- 23.Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 24.Harries AD, Lin Y, Satyanarayana S, Lönnroth K, Li L, Wilson N, et al. The looming epidemic of diabetes-associated tuberculosis: Learning lessons from HIV-associated Tuberculosis. Int J Tuberc Lung Dis. 2011;15:1436–44. doi: 10.5588/ijtld.11.0503. [DOI] [PubMed] [Google Scholar]


