Figure 1.
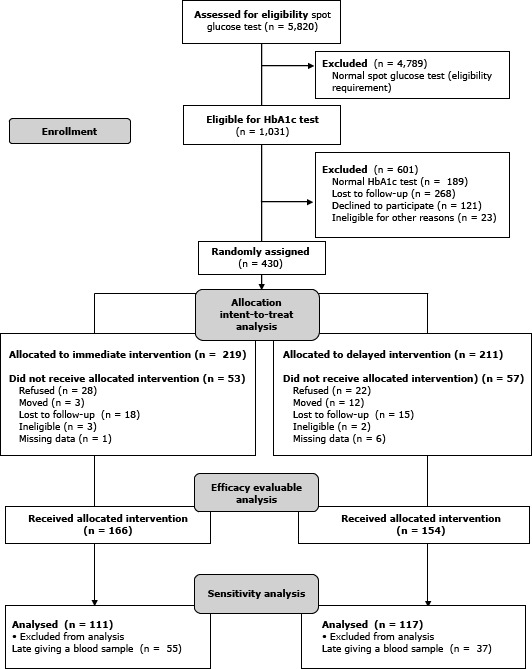
Overview of the process for the randomized-controlled prevention trial of the Partnership for a Hispanic Diabetes Prevention Program.

Overview of the process for the randomized-controlled prevention trial of the Partnership for a Hispanic Diabetes Prevention Program.