Fig. 2.
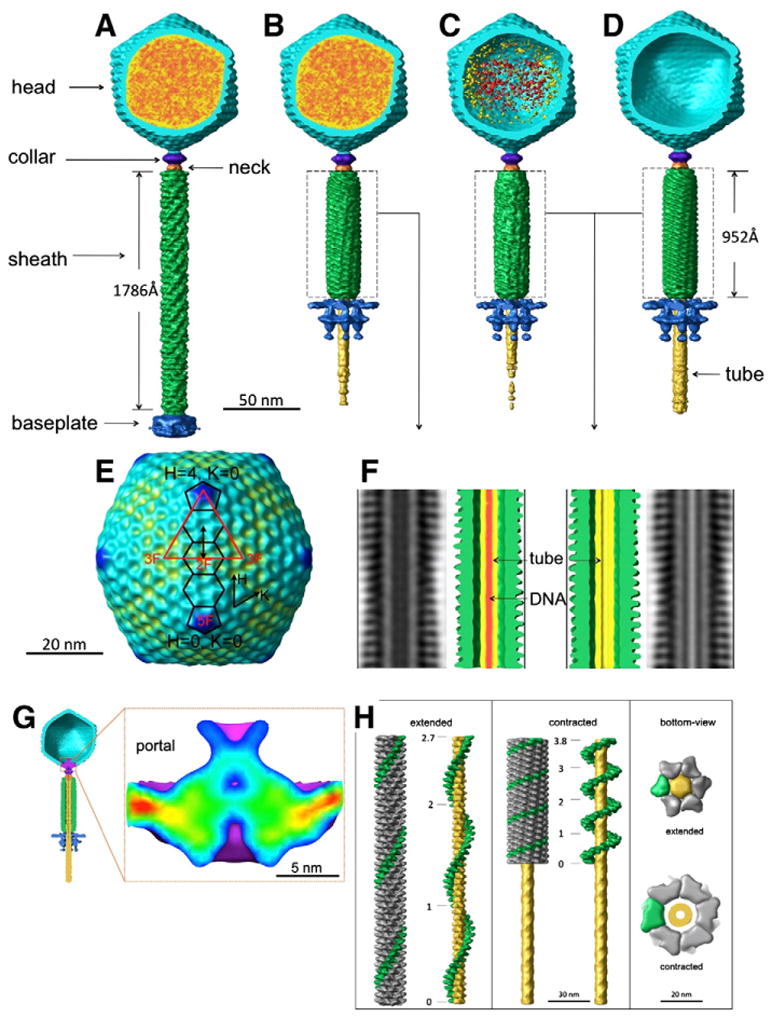
Three dimensional reconstructions of four distinct states of SBP8a (A–D). The head is colored in cyan, DNA in red and orange, the tail in green, the baseplate in blue, the tail tube in yellow, the collar in purple, and the neck in orange. A reconstruction of the SBP8a capsid with icosahedral symmetry imposed is shown in E. The icosahedral asymmetric unit is shown as a red triangle with 5-, 3- and 2-fold symmetry axes labeled. Two pentamers and three hexamers along the H-direction are outlined in black. The approximately 140 Å distance between neighboring hexamers is shown by a double-headed black arrow. F shows helical reconstructions of the contracted tails from B (left) and C, and D (right) respectively. DNA is shown as red density in the tail tube on F (left). G. Structure of the SBP8a portal. A cut-away view of the phage particle with an empty head and contracted tail is shown, with density corresponding to the portal protein colored in purple (left). A close-up of the portal of SBP8a, colored blue (low) to red (high) by density height is shown in the panel on the right. H. Two conformations of the tail sheath. The reconstructions of the extended tail (left) and contracted tail (middle) were carried out using helical reconstruction techniques modified for tomographic data. One of the six helical strands is colored in green. The tail tube in the middle of the tail sheath is colored in yellow. Total twist angles are shown along a single-strand of the sheath. Bottom-views of extended and contracted tails are shown in the right panel.
