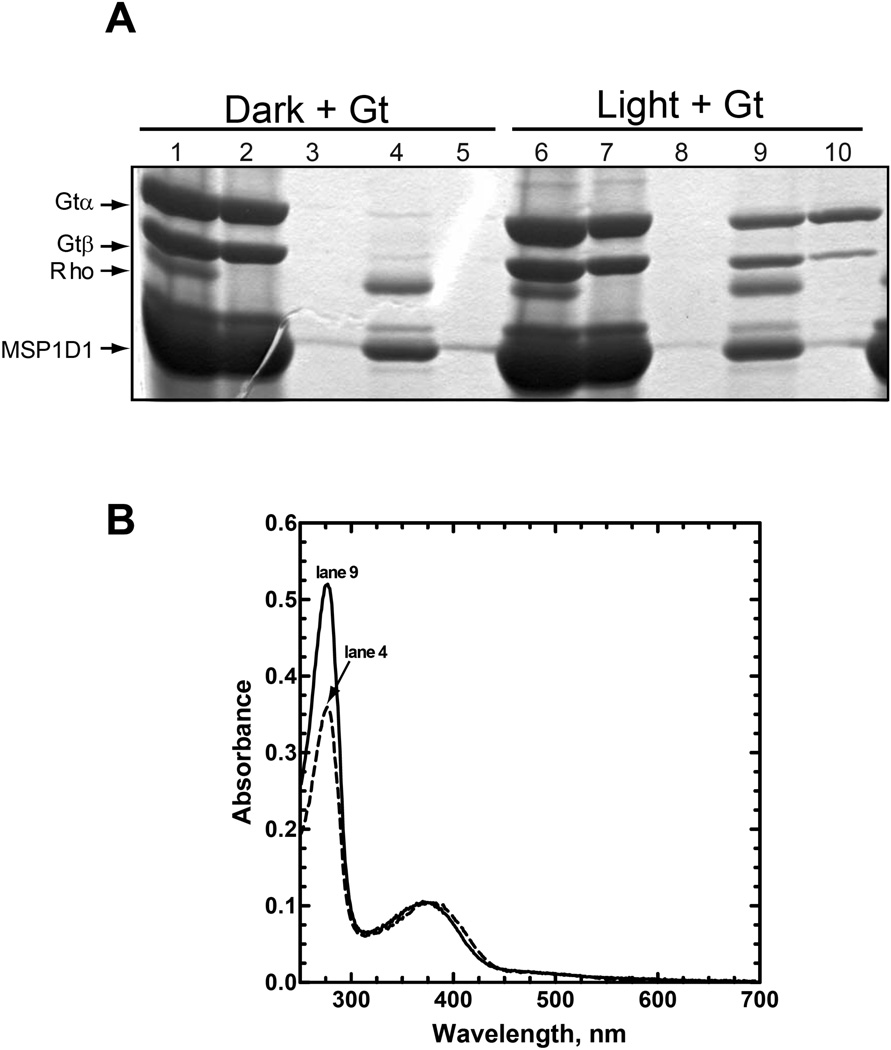
Figure 3.
Preparation and purification of an R*/Gt complex. Rhodopsin reconstituted with 11-cis-retinal in Nanodiscs was mixed with a 2-fold excess of Gt and then either incubated in the dark or illuminated for 2 min with light from a 300W tungsten bulb, as indicated in the figure. The mixture was then applied to a 1D4-Sepharose immunoaffinity column for purification as described in the Experimental Procedures. (A) Commassie Blue-stained SDS-PAGE gel containing samples from each step in the purification procedure. Lanes 1–5, sample in the dark; lanes 6–10, sample exposed to light. Lanes 1 & 6, material loaded onto the 1D4-Sepharose column; lanes 2 & 7, unbound material; lanes 3 & 8, last wash; lanes 4 & 9, 1D4-eluate; and lanes 5 & 10, GTPγS eluate. Only the α- and β-subunits of Gt are shown; in this and subsequent figures, the γ-subunit is not shown because it runs off the end of the gel. Molecular weights are as follows (Da): Gtα, 39,966; Gtβ, 37,377; Rho (N2C,E113Q,D282C mutant), 38,984; and MSP1D1, 24,662. The band migrating just above MSP1D1 on the gel is an impurity from the MSP1D1 preparation and has not been identified. As is noted in the text, the lower yield of the Gtβ-subunit from the GTPγS-elution, here (lane 10) and in Fig. 7, presumably reflects nonspecific binding of the more hydrophobic, isoprenylated γ-subunit to immobilized Nanodiscs on the column. (B) UV-visible absorption spectra for the purified samples from lanes 4 and 9 from Panel A, as is indicated in the figure.