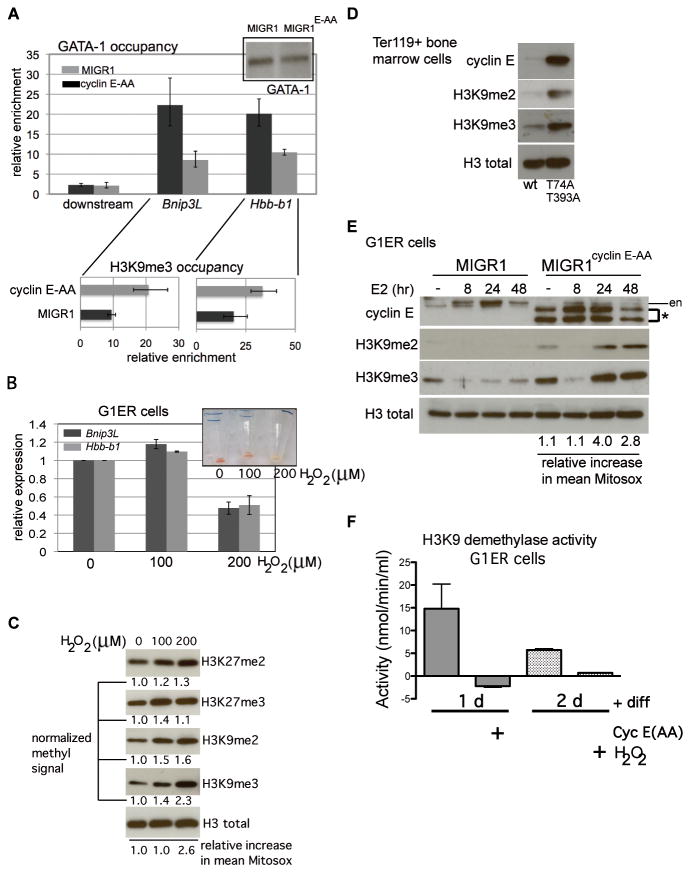
Figure 7. Increased reactive oxygen species, induced by cyclin E or exogenously administered, induce histone H3 lysine 9 hyper-methylation in differentiated erythroid cells and disrupt GATA-1 interactions with target genes.
(A) Chromatin immunoprecipitation (ChIP) analysis of GATA-1 occupancy at the Hbb-b1 promoter and Bnip3L (first intron) and a 10kb-downstream region was performed in G1ER cells transduced with the indicated constructs and induced for differentiation for 24 hours. Enrichment, relative to IgG control, is shown for one representative of three independent sets of transductions and ChIPs. Corresponding enrichment for tri-methylated H3K9 (H3K9me3) at each gene is displayed below GATA-1 occupancy data. (B) Expression of Hbb-b1 and Bnip3L was measured in differentiated G1ER cells, following treatment with hydrogen peroxide (H2O2) at the indicated doses. Exogenous peroxide was added eight hours after induction of differentiation with beta-estradiol, and cells were collected at 48 hours. Non-viable cells were excluded from collection by detecting retained propidium iodide. Inset shows cell pellets with H2O2 administration at the indicated doses (in μM). (C) Lysates were prepared from differentiated G1ER cells, following treatment with hydrogen peroxide (H2O2) for immunoblot assays. Quantitation of each histone modification was performed with normalization to total H3 signal. Shown is a representative result from three independent experiments. Mean increases in Mitosox signal relative to untreated cells were obtained using FlowJo and are expressed relative to untreated control. (D) Ter119+ bone marrow cell lysates from mice of the indicated genotypes were electrophoresed and immunoblotted as shown. Total H3 is shown as loading control. Displayed data are representative of three independent experiments. (E) G1ER cells, transduced as shown, were collected at the indicated time points during erythroid differentiation then lysed for immunoblot analyses. (en – endogenous cyclin E signal, * - retrovirally expressed cyclin E). Mitochondrial superoxide levels during differentiation were measured as for Figure 4D and mean Mitosox signal calculated for cyclin E-AA-transduced cells is expressed relative to the corresponding MIGR1-transduced cell time point. (F) Endogenous H3K9 demethylation activity was measured in lysates prepared from G1ER cells transduced or treated as indicated after differentiation in culture for the indicated times, using a quantitative fluorometric assay. Cyclin E(AA)-transduced cell lysates yielded readings above substrate-free baseline readings, though activity calculated using formula described in Methods obtains negative value. Results displayed are from a representative assay of two independent experiments.