Abstract
Human endogenous retrovirus-H (HERV-H) is implicated in leukaemias and lymphomas, but the precise molecular mechanism underlying HERV-mediated carcinogenesis remains unknown. We determined the prevalence of HERV-H in a cross-section of the Singapore population and explored the relationship between HERV-H positivity and incidence rates for Hodgkin's lymphoma in three major ethnic groups of Singapore. We observed that Malays were 1.11 times likely (95% CI=1.05–1.17; P<0.01), and Indians 1.12 times likely (95% CI=1.07–1.18; P<0.01) to be HERV-H positive when compared to Chinese. Interestingly, the incidence rates of Hodgkin's lymphoma for the three races positively correlated to the respective prevalence rate for HERV-H positivity (r=0.9921 for male; r=0.9801 for female), suggesting that viral inheritance in human may predispose certain racial origin unfavourably to malignancy.
Keywords: HERV, Lymphoma, Ethnicity, Prevalence, Singapore
1. Introduction
Leukaemias, lymphomas and multiple myeloma can be the dreadful sequelae of viral infections. RNA oncogenic viruses, which are also known as retroviruses, were initially known to cause lymphoproliferative disorders only in animals, but have also been reported to demonstrate their transforming ability in humans. Retroviruses are capable of integrating their proviral DNA into the host genome, which may in turn, mediate leukemogenesis via insertional mutagenesis. For instance, human T-cell lymphotropic virus type 1 is the causative agent of adult T-cell leukaemia–lymphoma [1]. Akin to exogenous retroviruses, human endogenous retroviruses (HERVs) have integrated into the human genome since at least 30 million years ago and are transmitted vertically via the germ line [2]. Some family members of HERVs (HERV-K and HERV-H) are implicated in leukaemias and lymphomas, although the precise molecular mechanism underlying HERV-mediated carcinogenesis remains to be elucidated [3]. While seroprevalence to exogenous viral infections in endemic regions have been shown to be associated with the incidence rate of lymphoid cancers [1,4], information about the prevalence of HERV-H in different populations in relation to lymphoid neoplasms remains unknown. Of interest, a recent study reported that endogenous LTR derepression is involved in the pathogenesis of Hodgkin's lymphoma [5]. This provide the impetus for us to determine the prevalence of HERV-H in the three major ethnic groups (Chinese, Malays and Indians) residing in Singapore, and examine the association between the prevalence rate of each of these ethnic group with the reported incidence rate of Hodgkin's lymphoma retrieved from the Singapore Cancer Registry.
2. Materials and methods
2.1. Study design and subjects
To investigate the prevalence of HERV-H in a cross-section of the Singapore population, 808 apparently healthy subjects, aged 16–60 years, were randomly recruited from the residents in Singapore during April 2010–November 2011. The mean age at recruitment was 23.3 years. Of these, 23 subjects whose genomic materials were classified as indeterminate were excluded from the study, resulting in a total number of 785 subjects.
The study protocol was approved by the Ethics Review Committee of Singapore Polytechnic.
2.2. Genotype analysis
Buccal cells were collected from volunteers using the MasterAmp™ buccal brush (Epicentre, USA) and their genomic DNA was isolated using the ReliaPrep gDNA Tissue Miniprep System (Promega, USA). Each individual's DNA was tested for the presence of HERV-H element by using polymerase chain reaction (PCR) amplifications as previously described [6]. Briefly, PCR was conducted using the primers HERV-H: forward, 5′-CTT CCC TCC GTG TCT TTA CG-3′ and reverse, 5′-AAG ATT AGA CAC ACT CAG CAA CG-3′. The PCR mix (volume of 50 μl) contained 20 ng of extracted genomic DNA template, 2.0 μM of each primer, 200 μM of each deoxynucleoside triphosphate, 15 mM of MgCl2, and 2.5 units of GoTaq® DNA polymerase (Promega, USA). PCR amplification was conducted with 2 min of initial denaturation at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at annealing temperature at 60 °C, and 60 s of elongation at 72 °C, followed by a 10 min final extension at 72 °C.To monitor for reagent contamination, a no template control (NTC) was included in every batch of PCR amplification. A housekeeping gene, β-actin, was used to validate the true negatives.
2.3. Statistical methods
Analyses were conducted separately for gender and ethnicity. Prevalence rates and 95% confidence intervals (95% CIs) were calculated using the ProMESA software, version 1.62 (EpiCentre, Massey University, New Zealand). Relative risks and odds ratios (ORs) with 95% CIs were calculated using the GraphPad Prism Version 6.0c (GraphPad Software, USA) to estimate the association of HERV-H with the variables. Differences between groups were estimated by chi-square test (statistical significance for P<0.05). Odds ratios statistical tests were based on 2-sided probability. Correlations between incidence rates and relative risks were calculated using Pearson's correlation (GraphPad Prism).
3. Results
Of 808 subjects recruited, 785 were successfully analysed for HERV-H element. The prevalence rates of HERV-H positivity, based on gender as well as categorised by their ethnic descent, are shown in Table 1. 89.9% (381/427) females and 88.6% (320/361) males were found to possess HERV-H in their genome, resulting in a total of 89.3% HERV-H positivity overall. With respect to gender, there is no statistical difference between males and females (P>0.05).
Table 1.
HERV-H positivity in the study Singapore population.
| HERV-H positive |
P value | HERV-H negative |
Relative risk | Odds ratio | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence rate (%) | 95% CIb | Difference (95% CI) | n | Prevalence rate (%) | 95% CIb | |||||
| Total | 701 | 89.3 | 87.1–91.5 | N.A. | N.A. | 84 | 10.7 | 8.5–12.9 | N.A. | N.A. | |
| Gender | |||||||||||
| Male | 320 | 88.6 | 86.4–90.8 | Reference | 41 | 11.4 | 9.2–13.6 | 1.00 | 1 | ||
| Female | 381 | 89.9 | 87.8–92.0 | 1.3 (−1.76 to 4.36) | 0.583 | 43 | 10.1 | 7.99–12.2 | 1.01 | 1.14 (0.72–1.79) | 0.583 |
| Race/ethnicity | |||||||||||
| Chinese | 438 | 86.1 | 83.7–88.5 | Reference | Reference | 71 | 13.9 | 11.5–16.3 | Reference | 1 | Reference |
| Male | 185 | 83.7 | 81.1–86.3 | 36 | 16.3 | 13.7–18.9 | Reference | 1 | Reference | ||
| Female | 253 | 87.8 | 85.5–90.1 | 35 | 12.2 | 9.91—14.5 | Reference | 1 | Reference | ||
| Malay | 124 | 95.4 | 93.9–96.9 | 9.3 (6.47–12.1) | 0.0035 | 6 | 4.6 | 3.13–6.07 | 1.11 (1.05–1.17) | 3.35 (1.42–7.89) | 0.0037 |
| Male | 68 | 95.8 | 94.4–97.2 | 3 | 4.2 | 2.8–5.6 | 1.14 (1.06–1.23) | 4.41 (1.32–14.8) | 0.0149 | ||
| Female | 56 | 94.9 | 93.4–96.4 | 3 | 5.1 | 3.56–6.64 | 1.08 (1.00–1.16) | 2.58 (0.77–8.70) | 0.1131 | ||
| Indian | 121 | 96.8 | 95.6–98.0 | 10.7 (7.98–13.42) | 0.0009 | 4 | 3.2 | 1.97–4.43 | 1.12(1.07–1.18) | 4.90 (1.76–13.7) | 0.0011 |
| Male | 56 | 98.2 | 97.3–99.1 | 1 | 1.8 | 0.87–2.73 | 1.17(1.10–1.26) | 10.9 (1.46–81.3) | 0.0035 | ||
| Female | 65 | 95.6 | 94.2–97.0 | 3 | 4.4 | 2.97–5.83 | 1.09 (1.02–1.16) | 3.00 (0.89–10.1) | 0.0629 | ||
| Othera | 18 | 85.7 | 83.3–88.2 | 0.4 (−3.04–3.84) | 0.9652 | 3 | 14.3 | 11.9–16.8 | 0.996 (0.83–1.19) | 0.97 (0.28–3.39) | 0.9652 |
Other (Burmese, Ceylonese, Eurasian, Filipino, Japanese, Javanese, Pakistani, Punjabi, Sikh, Vietnamese).
95% CI: 95% confidence interval; calculated based on Singapore Population in 2011 i.e., 5,183,700.
Source: Singapore Department of Statistics.
The prevalence rates of HERV-H positivity for Chinese, Malays and Indians are 86.1%, 95.4%, and 96.8%, respectively. To examine the significance of ethnic disparities, we performed a Chi-square test. Compared to Chinese, both Malays and Indians were found to possess statistically higher prevalence rates (P<0.01). In addition, Malays were also found to be 1.11 times likely (95% CI=1.05–1.17; P<0.01), and Indians 1.12 times likely (95% CI=1.07–1.18; P<0.01) to be HERV-H positive when compared to Chinese.
Fig. 1 depicts the strong association between the Hodgkin's lymphoma incidence rates and the prevalence rate for HERV-H positivity in three major ethnic groups of Singapore (1A: r=0.9921 for male; 1B: r=0.9801 for female). The age standardised rate risks data was obtained from the Singapore Cancer Registry Report No. 7 [7].
Fig. 1.
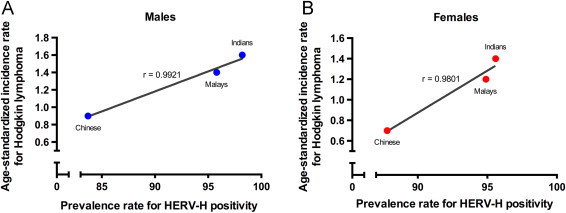
Correlation between Hodgkin lymphoma age-standardized incidence rates [7] HERV-H positivity in three major ethnic groups of Singapore. Regression lines are plotted to show a strong association between age-standardized incidence rates and prevalence rates in males (A) and females (B) (r=0.9921 for males; r=0.9801 for females).
4. Discussion
HERVs represent the trail of successful ancient retroviral infection of the human germ line. As a consequence to the persistence of vertical transmission, about 8% of the human genome is occupied by HERVs [2]. Their existence, through millions of years of evolution, implies that HERVs have overcome the stringent evolutionary selection and their genomes have been successfully domesticated and incorporated into the host genomes, although the accumulative effects of mutations, deletions, frame shifts, premature stop codons and hypermethylation of the promoter sites may have rendered them defective or inactive. While retroelements such as Alu (in SINE) is implicated in pathogenesis of autoimmune lymphoproliferative syndrome and X-linked agammaglobulinemia, and L1 (in LINE) in haemophilia A and B [8], the role of HERV in carcinogenesis remains poorly understood.
Interestingly, our analysis reveals strong positive correlation between age standardized incidence rate of Hodgkin's lymphoma and prevalence rate for HERV-H positivity. Based on genetic similarity in the pol region, HERV-H belongs to Class I of the Gammaretrovirus-like family. Apart from possessing long terminal repeats (LTR)s, HERV-H is also known to utilise histidine (H) transfer RNA (tRNA) at its primer binding site to initiate reverse transcription [2]. A previous study demonstrated that derepression of endogenous LTR due to hypomethylation results in up-regulation of CSF1R proto-oncogene expression, which in turn contributes to the pathogenesis of Hodgkin's lymphoma [5]. In addition, endogenous retroviral Np9 and Rec were identified as oncogenic players in leukaemia [2]. Expression of HERV-H was also shown to contribute to pluripotency in human cells [9]. Perturbation to HERV-H-mediated pluripotency regulation may lead to deregulation in self-renewal control and emergence of cancer stem cell, though this hypothesis remains to be proven.
In contrast to the low insertion frequency in Asian population for HERV-K113 (13%) and -K115 (4%) [10], we demonstrate herein high insertion frequency (89.3%) of HERV-H in the Singapore population, and observe interesting correlation between prevalence and age-standardized incidence rate of Hodgkin's lymphoma for the three races residing in Singapore. Together, current and other study [10] provides the differential prevalence rate of various HERV elements in different human race, and can serve to demonstrate these “jumping genes” retrotransposons (e.g., HERV-H vs. HERV-K) have vastly different distribution frequency. Further prospective or retrospective HERV-H gene expression studies involving cohort of Hodgkin's lymphoma patients are required to directly implicated endogenous retroviral elements in lymphomagenesis and investigate the molecular mechanisms underlying the process.
Authors' contributions
W.H.W. performed experiments. W.H.W., L.S., S.M.L. and E.S-C.K. interpreted the data. W.H.W. designed the study and drafted the manuscript. S.M.L. and E.S-C.K. revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Acknowledgements
This work was supported by Grants from Singapore Polytechnic [Grant numbers 11–27801-45–2214, 11-27801-45-2554, 11-27801-45–2762 and 11–27801-45–3286].
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Proietti F.A., Carneiro-Proietti A.B., Catalan-Soares B.C., Murphy E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 2.Bannert N., Kurth R. Retroelements and the human genome: new perspectives on an old relation. Proc Natl Acad Sci USA. 2004;101(Suppl. 2):14572–14579. doi: 10.1073/pnas.0404838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanish M.T., Cohen C.J., Mager D.L. Potential mechanisms of endogenous retroviral-mediated genomic instability in human cancer. Semin Cancer Biol. 2010;20:246–253. doi: 10.1016/j.semcancer.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Magrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. Br J Haematol. 2012;156:744–756. doi: 10.1111/j.1365-2141.2011.09013.x. [DOI] [PubMed] [Google Scholar]
- 5.Lamprecht B., Walter K., Kreher S., Kumar R., Hummel M., Lenze D. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16:571–579. doi: 10.1038/nm.2129. [DOI] [PubMed] [Google Scholar]
- 6.Alves P.M., Levy N., Stevenson B.J., Bouzourene H., Theiler G., Bricard G. Identification of tumor-associated antigens by large-scale analysis of genes expressed in human colorectal cancer. Cancer Immun. 2008;8:11. [PMC free article] [PubMed] [Google Scholar]
- 7.National Registry of Diseases Office. Trends in cancer incidence in Singapore 1968–2007. Singapore Cancer Registry Report No. 7. Singapore Cancer Registry, Singapore; 2010.
- 8.Solyom S., Kazazian H.H., Jr Mobile elements in the human genome: implications for disease. Genome Med. 2012;4:12. doi: 10.1186/gm311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoni F.A., Guerra J., Luban J. HERV-H RNA is abundant in human embryonic stem cells and a precise marker for pluripotency. Retrovirology. 2012;9:111. doi: 10.1186/1742-4690-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha A.R., Pillai S.K., York V.A., Sharp E.R., Storm E.C., Wachter D.J. Cross-sectional dating of novel haplotypes of HERV-K 113 and HERV-K 115 indicate these proviruses originated in Africa before Homo sapiens. Mol Biol Evol. 2009;26:2617–2626. doi: 10.1093/molbev/msp180. [DOI] [PMC free article] [PubMed] [Google Scholar]


