Figure 4.
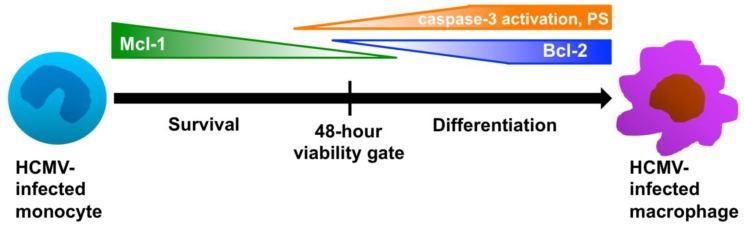
HCMV regulates the distinct processes of survival and differentiation during infection through the precise expression of specific pro- and anti-apoptotic molecules. Our data have shown that HCMV infection induces the enhanced expression of Mcl-1 in infected monocytes in order to mediate their prolonged survival through a 48-hour viability checkpoint, a time in which uninfected monocytes are programmed to die [24,25]. Mcl-1 levels gradually decrease through 48 hours after infection, before becoming undetectable. Following 48 hours, HCMV induces the enhanced expression of Bcl-2 and phosphatidyl serine (PS) membrane expression and an increase in the partial activation of caspase-3 in infected monocytes. The expression of low levels of Bcl-2 helps to support the survival of infected monocytes after the 48-hour viability gate, while precise regulation of the membrane expression of PS and the partial activation of caspase-3 help to drive the differentiation of infected monocytes towards replication-permissive macrophages. Overall, through the tight regulation of pro- and anti-apoptotic molecules, HCMV is able to overcome critical biological hurdles, such as the short lifespan of monocytes and the limit of de novo viral gene expression and/or replication in monocytes.