Figure 2. Organization of the scaffolding domains.
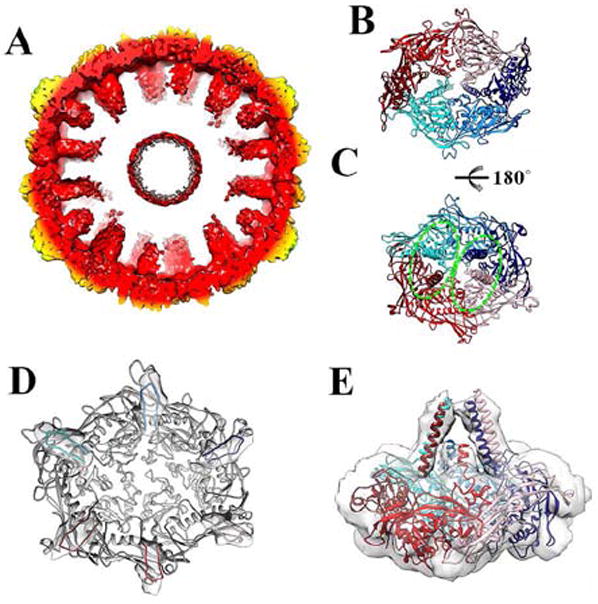
(A) Cross-section of the protease-free Prohead-1 reconstruction revealing the scaffolding domains extending toward the procapsid center. The reconstruction is radially colored as in Figure 1. (B) Each Prohead-1 hexon (viewed from the capsid exterior) can be divided into two-halves according to the position of the coat subunit cores (delimited by the two shades of red and blue). (C) The scaffolding domains belonging to each Prohead-1 hexon (viewed from the capsid interior) can also be divided into two groups (delimited by the dashed ellipsoids) that differ from the ones formed by the coat subunit cores. (D) The coat subunit N-arms (residues 104-130) lie on the internal face of each capsomer and connect to the scaffolding domains (viewed from the capsid interior). The N-arms are colored by subunit identity and depicted within the corresponding region of the reconstruction while the rest of the subunits are colored grey. (E) Lateral view of a Prohead-1 hexon fitted into the reconstruction to show the approximate organization of the scaffolding domain C-terminal moieties (residues 80-104) relative to the coat subunits. The coat subunit coordinates are colored as in Figure 2.
