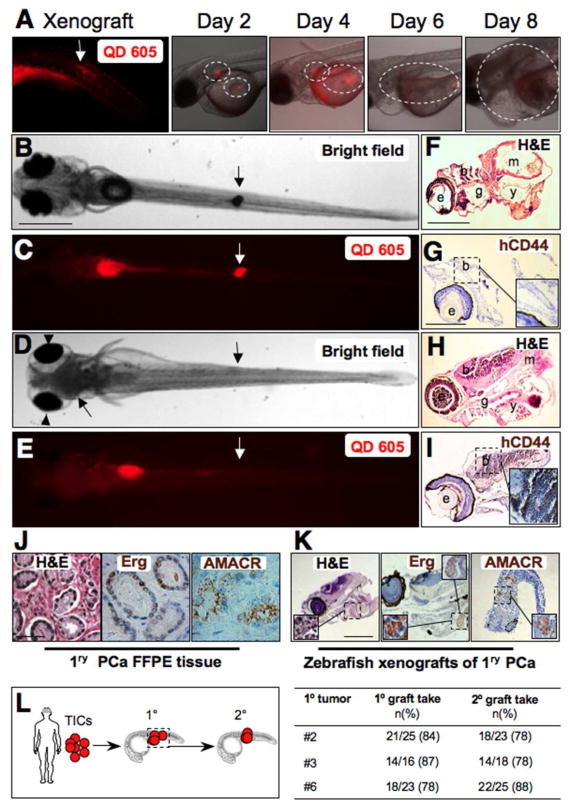
Fig. 5.
Prostate cancer xenograft tumor progression in zebrafish. A: QD 605 red fluorescent image of the site of injection on the left taken on the same day of transplanting primary PCa cells SC in zebrafish embryos. Red and bright image overlays represent sequential imaging of the same xenograft embryo over time during 8 days of tumor growth. From day 2, notice the two sites that are outlined with tumor cells labeled with QD. Tumor growth increases progressively while fluorescence decreases due to QD dilution with cell division. B–E: Sections from control and PCa xenografts with brain metastasis (D–E). Images were taken at 9 dpt. Downward arrows indicate the site of SC transplantation. Upward arrows in D and E on the left indicate cell masses invading the brain and causing exophthalmus (arrowheads in D, compare with no brain metastasis in B). F–K, H&E staining (F and H) and IHC with anti-human CD44 (G and I) in a representative control untransplanted embryo (F–G) compared to histological sections from a representative xenotransplanted embryo (H–I) with brain metastasis stained with H&E (H) and IHC staining of the same brain region containing disseminated human cells identified with the human isoform-specific anti-CD44 antibody (Insert) (H,I). Letters indicate e, eye; b, brain; m, muscle; y, yolk. Inserts in panels G and I are higher magnification of the outlined brain areas, respectively. J: Formalin fixed paraffin embedded (FFPE) sections from a representative primary prostate cancer (PCa) tissue used that are stained with H&E, or in IHC with either Erg or AMACR in brown. The H&E image in J is a higher magnification of the outlined areas in supplementary Fig. 4C, while The Erg and AMACR images in J are higher magnifications of the outlined areas in supplementary Fig. 6H and 5F, respectively. K: Sections from zebrafish embryo with PCa xenograft using cells from tissues shown in Fig. 5K and stained with H&E, and IHC for Erg and AMACR. Inserts are higher magnification of the outlined area in K. L: Diagram demonstrating strategy to study tumor initiation potential of primary PCa cell grafts in secondary xenografts. TICs from the 5-min adherent α2β1hi/CD44hi cells of three patient samples #4, #5, and #6 were transplanted to generate primary xenografts (1°). Xenograft tumor areas were dissected, pooled, and TICs were sorted and injected into secondary recipients. Table on the right demonstrates primary and secondary graft take rates. Scale bars are 100 μm in B–E, F–J, and N–K.