Abstract
Objectives
The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (“statins”) have anti-inflammatory properties and are associated with improved outcomes in critically ill patients. We investigated whether previous statin therapy affects outcomes in patients at risk for acute respiratory distress syndrome.
Design
Patients were followed-up for the primary outcome of acute respiratory distress syndrome and secondary outcomes of intensive care unit and 60-day mortality, organ dysfunction, and ventilator-free days in a secondary analysis of a prospective cohort study. Receipt of statin therapy was recorded. Propensity score matching was used to adjust for confounding by indication.
Setting
Intensive care units at a tertiary care academic medical center.
Patients
Critically ill patients (2,743) with acute respiratory distress syndrome risk factors.
Interventions
None.
Measurements and Main Results
Acute respiratory distress syndrome developed in 738 (26%) patients; 413 patients (15%) received a statin within 24 hrs of intensive care unit admission. Those who had received a statin within 24 hrs had a lower rate of development of acute respiratory distress syndrome (odds ratio 0.56; 95% confidence interval 0.43–0.73; p < .0001). After multivariate adjustment for potential confounders, this association remained significant (odds ratio 0.69; 95% confidence interval 0.51–0.92; p = .01). However, after propensity score matching, the association was not statistically significant (odds ratio 0.79; 95% confidence interval 0.57–1.10; p = .16). Statin use was not associated with reduced acute respiratory distress syndrome mortality, organ dysfunction, or ventilator-free days. Results of the study were presented in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Conclusions
Statin therapy at the time of intensive care unit admission was not associated with a lower rate of development of acute respiratory distress syndrome after matching for patient propensity to receive statins. Statin therapy was not associated with improvements in acute respiratory distress syndrome mortality, organ failure, or days free from mechanical ventilation.
Keywords: ALI/ARDS, critical illness, statin
A Cute respiratory distress syndrome (ARDS) is a devastating syndrome characterized by diffuse alveolar damage resulting from inflammation, endothelial damage, and fibroproliferative remodeling in the lungs that occurs after an inciting injury (1). More than 150,000 cases and >70,000 deaths from ARDS are estimated to occur in the United States each year (2). As yet, there is no effective pharmacologic therapy for treatment of ARDS after decades of research (3).
Inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, also known as “statins,” are effective for the treatment and prevention of coronary heart disease (4). Statins exert pleiotropic anti-inflammatory effects in addition to their lipid-lowering effect (5). Accordingly, there is great interest in utilizing statins not only as a therapy for inflammatory diseases such as septic shock and ARDS but also for prevention of inflammatory complications such as ARDS after injury (6). Preliminary data from animal models of lung injury support these approaches (7–9) and observational studies report improved outcomes among patients who received statins before the development of pneumonia, sepsis, and other inflammatory or infectious conditions (10–13). However, data supporting improved outcomes in human lung injury are lacking (14, 15), although recent studies have suggested a potential protective effect of statins against lung injury (16, 17), and a phase II treatment study in patients with lung injury has been conducted (18).
Because of the potential promise of statin therapy in preventing and treating ARDS, we conducted an observational study of a large cohort of patients at risk for ARDS. We sought to evaluate whether patients receiving statins are at reduced risk for development of ARDS and whether they have better outcomes when ARDS develops.
MATERIALS AND METHODS
Study Design and Enrollment
From September 1999 to March 2009, we screened all patients admitted to the surgical, medical, and neurosciences adult intensive care units (ICU) at Massachusetts General Hospital prospectively for risk factors for ARDS, including pneumonia, sepsis, septic shock, aspiration, trauma, and multiple transfusions (Table 1). Both inclusion and exclusion criteria are as previously described and included immuno-suppressed patients and those with comfort care orders (19). We enrolled patients with ARDS risk factors and no exclusion criteria after informed written consent was obtained from them or a surrogate. Study procedures were approved by the Institutional Review Board of our institution.
Table 1.
Risk factors for ARDS required for study inclusion and exclusion criteria considered
| Risk Factor | Definition |
|---|---|
| Sepsis | Known or suspected source of systemic infection plus at least two of the following: a) temperature >38°C or <36°C; b) heart rate >90 beats/min; c) respiratory rate >20 breaths/min or PaCOC <32 mm Hg; d) WBC count >12,000/mm3, <4,000/mm3, or >10% bandemia. |
| Septic shock | Fulfill requirements for sepsis plus one of the following: a) SBP <90 mm Hg or a reduction of ≥40 mm Hg from baseline for ≥30 mins, unresponsive to 500 mL of fluid resuscitation; b) need for vasopressors to maintain systolic blood pressure SBP ≥90 mm Hg or within 40 mm Hg of baseline. |
| Pneumonia | Fulfill two or more of the following: a) new airspace opacity on chest radiograph; b) temperature >38.3°C or <36.0°C, white blood cell > 12,000/mm3 or <4,000/mm3 or >10% bandemia; c) positive microbiological culture. |
| Aspiration | Defined as witnessed or documented aspiration event or the retrieval of gastric contents from the oropharynx, endotracheal tube, or bronchial tree. |
| Trauma | Defined as multiple fractures and/or pulmonary contusions. Multiple fractures are defined as a fracture of two long bones, an unstable pelvic fracture, or one long bone and a pelvic fracture. Pulmonary contusion is defined as airspace opacity on chest radiograph within 8 hrs of admission to the emergency room and evidence of blunt trauma to the chest, for example, fractured ribs or ecchymosis overlying airspace opacity. |
| Multiple transfusion | Defined as receiving ≥8 units of packed red blood cells within 24 hrs |
| Exclusion criteria | |
| 1. Age < 18 yrs | |
| 2. Diffuse alveolar hemorrhage | |
| 3. Chronic lung diseases other than chronic obstructive pulmonary disease or asthma that may preclude diagnosis of ARDS | |
| 4. Directive to withhold intubation or decision to withdraw aggressive ICU care | |
| 5. Immunosuppression not secondary to corticosteroid | |
| 6. Treatment with immunomodulation agents such as granulocyte colony-stimulating factor or inhibitors of tumor necrosis factor | |
PaCO2, arterial carbon dioxide tension; WBC, white blood cell; SBP, systolic blood pressure; ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Data Collection
We collected demographic data at baseline, including data used for Acute Physiology and Chronic Health Evaluation (APACHE) III score calculation, which includes components of chronic health status (20), and accessed inpatient and outpatient records to determine whether patients were prescribed statins before hospital admission and whether they received doses of statins while in the hospital. We screened patients daily while in the ICU for development of ARDS. The primary outcome was development of ARDS as defined by American-European Consensus Committee criteria (21) and requirement for mechanical ventilation. Daily chest radiographs were performed according to our institution’s protocol for management of mechanically ventilated patients. Radiographs were independently interpreted by study staff comprising critical care-trained physicians who were not involved with care of the patient. All study staff were required to complete a standardized computer training module for interpreting ARDS chest radiographs. For quality-control purposes, films were periodically selected for reading by multiple study staff: testing showed a high degree of concordance between readers (κ > 0.8). Study staff alternated reading patient radiographs on sequential days, and in cases of discrepant readings another investigator was available for adjudication. We followed-up all patients for the secondary end point of ICU mortality. Among patients who had ARDS develop at any time, including those who presented with ARDS on hospital day 1, we screened them daily for secondary study end points, including 60-day mortality, daily multiple organ dysfunction score (MODS) calculated by Brussels criteria (22), and number of days free from mechanical ventilation at 28 days. Data regarding receipt of statin therapy were collected retrospectively after the prospective cohort had been established.
The rates of missing data were low (<1%). If patients were missing data required for a particular analysis, then they were excluded from that analysis.
Statistical Analysis
We performed all statistical analyses using SAS software version 9.1.3 (SAS Institute Inc, Cary, NC) and considered p < .05 to be statistically significant. We used chi-squared tests, two-sample t tests, or Wilcoxon rank-sum tests as appropriate for univariate analyses. We stratified patients for comparison according to whether they had received any statin doses within 24 hrs before ICU admission.
To analyze the primary outcome of ARDS development and the secondary outcome of mortality, we used logistic regression modeling. We planned a priori to adjust these analyses for age and severity of illness (as measured by APACHE III score; the age component was subtracted from APACHE III scores to avoid colinearity between variables, and the PaO2/FIO2 component was removed from the APACHE III score in the multivariate models of ARDS risk to avoid colinearity with the outcome). We considered other variables for inclusion in the logistic regression models using a backward stepwise selection algorithm with p ≤ .1 as the cut-off for inclusion. These included other variables of clinical relevance, potential confounders, or those with significant differences between groups on univariate analysis. Specifically, we considered variables such as gender, race, vasopressor or corticosteroid use, number of red blood cell transfusions, presence of shock, liver, or renal failure, and history of diabetes, among others. Variables were included in the model if they survived stepwise selection. Because of a previous report suggesting that aspirin therapy was actually responsible for some of the preventive effect associated with statin use (16), we included this as a variable; however, it failed to meet the significance threshold for inclusion. We did attempt forcing it into the multivariable models as a covariate, but again it did not have a significant association with the outcome, nor did it substantially affect model fit, and ultimately aspirin use was not included in the final models. Similar proportions of patients received aspirin in the statin and nonstatin groups (53% and 47%, respectively). Thus, the final multivariable model for the primary and secondary outcomes included age, APACHE III score (modified as described), and other covariates as described herein. To account for changes in treatment over time, we also matched patients according to the calendar year in which the patient was enrolled using conditional logistic regression.
We then conducted a propensity score analysis in an effort to reduce bias introduced by nonrandom patient-associated factors for receipt of statin therapy. Using logistic regression modeling, we first constructed a propensity score for receipt of statin therapy. Variables for the propensity score were selected from among demographic and clinical variables associated with ARDS risk and with statin use on multivariate analysis, according to the methods of propensity score construction described by Brookhart et al for controlling confounding by including covariates that are related to the outcome as well as those related to the exposure (23). Covariate balance was tested within deciles of the propensity score and covariates were found to be well-balanced, indicating that the score was appropriately constructed. Details of this analysis and of covariates included in the final propensity score are described in the online supplement (see Supplemental Digital Content 1, http://links.lww.com/CCM/A395). After a propensity score for receipt of statins had been calculated for all subjects, we repeated multivariate analyses for the outcomes of interest in a matched logistic regression model using decile of propensity score as the matching variable, along with matching for calendar year as in the previous analyses. Additionally, we repeated the propensity score analysis by including computed propensity score as a covariate in the logistic regression model as an alternative to matching.
For the secondary outcomes, we modeled daily MODS, compared between groups, using a linear mixed-effects model (PROC MIXED in SAS 9.1.3) with age and admission APACHE III score included as fixed effects and daily MODS as repeated measures. We assumed an unstructured covariance matrix for the model. We used a generalized linear model including age and admission APACHE III score as covariates to compare the number of ventilator-free days between groups. We analyzed daily MODS and ventilator-free day models with and without propensity score included as a covariate.
Power
We calculated that with 2,330 patients in the nonstatin group and 413 patients in the exposure group, at α = .05 we had >80% power to detect a change of 5% in the proportion of patients with development of ARDS.
RESULTS
Primary Outcome
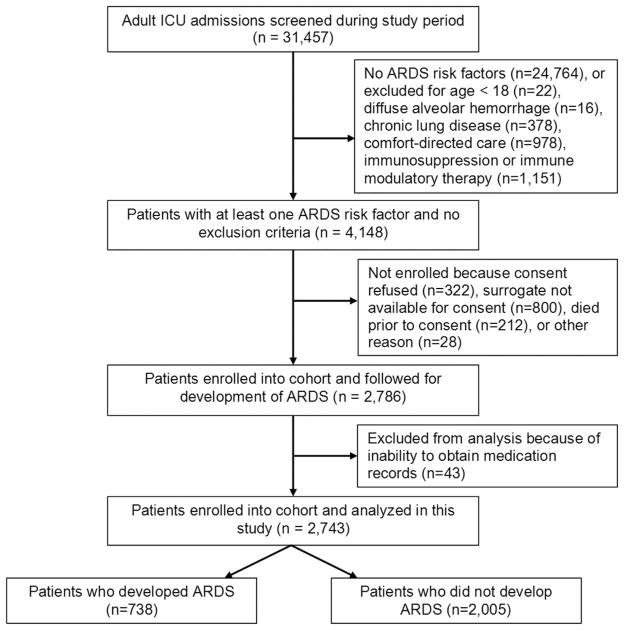
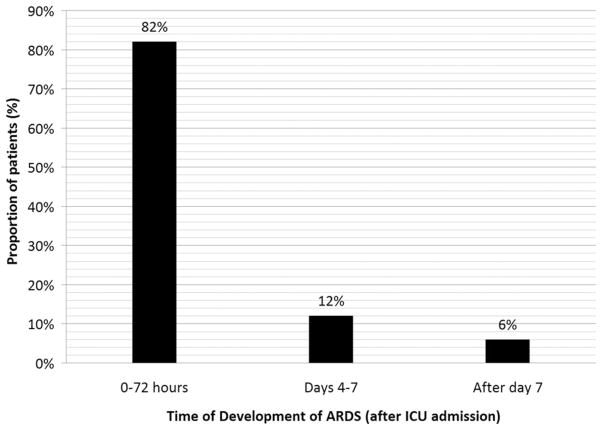
During the study period, we enrolled and collected data for 2,743 patients. Patient flow is depicted in Figure 1, including the number of patients screened and excluded; 346 patients (13%) died during admission. Median APACHE III score was 66 (25%–75% interquartile range, 34–98); 413 patients (15%) received at least one confirmed dose of statin medication within 24 hrs before ICU admission. The majority of these had been prescribed statins as outpatients; only ten patients received an entirely new prescription for statin therapy after admission and 607 patients (22%) had been prescribed statins chronically. Baseline characteristics of these patients are shown in Table 2; 738 patients had development of ARDS (27%). Of these, 75 patients (18%) had received statins. Patients in the statin group were significantly less likely to have development of ARDS (odds ratio [OR] 0.56; 95% confidence interval [CI] 0.43–0.73; p .01). Timing of development of ARDS is depicted in Figure 2.
Figure 1.
Participant flow diagram depicting screening/enrollment process. ARDS, Acute Respiratory Distress Syndrome; ICU, intensive care unit.
Table 2.
Baseline characteristics of study population
| Received Statin (n = 413) | Did Not Receive (n = 2330) | p | |
|---|---|---|---|
| Age (mean ± SD) | 68.4 (±12.3) | 58.1 (±18.2) | <.01 |
| APACHE III score mean (± SD) | 66.5 (±19.1) | 81.0 (±17.9) | <.01 |
| Female gender | 145 (38%) | 885 (38%) | .26 |
| Caucasian race | 391 (95%) | 2104 (90%) | <.01 |
| Pneumonia | 222 (54%) | 1177 (51%) | .22 |
| Sepsis without shock | 117 (28%) | 774 (33%) | .05 |
| Septic shock | 220 (53%) | 1126 (48%) | .06 |
| Trauma | 14 (3%) | 203 (9%) | <.01 |
| Aspiration | 32 (8%) | 180 (8%) | .99 |
| Direct lung injury (vs. indirect) | 233 (56%) | 1315 (56%) | .99 |
| Multiple transfusions | 34 (8%) | 247 (10%) | .14 |
| Corticosteroid use | 34 (8%) | 183 (8%) | .80 |
| Diabetes history | 182 (44%) | 493 (21%) | <.01 |
| BMI | 28.2 (± 7.4) | 27.7 (± 8.1) | .33 |
| Obese (BMI >30) | 88 (21%) | 439 (19%) | .24 |
| Morbidly obese (BMI >40) | 28 (7%) | 141 (6%) | .57 |
| Cirrhosis | 4 (1%) | 111 (5%) | <.01 |
| Hepatic failure | 6 (1%) | 57 (2%) | .20 |
| Ethanol abuse | 5 (1%) | 69 (3%) | .04 |
APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index.
Values represented as mean (± SD) for continuous variables and N (%) for categorical variables.
Figure 2.
Timing of development of acute respiratory distress syndrome (ARDS). ICU, intensive care unit.
Receipt of statins was again associated with significantly decreased odds of development of ARDS on an adjusted analysis (OR 0.69; 95% CI 0.51–0.92; p = .01). Full results of the multivariate analysis including all covariates are presented in Tables 3 and 4.
Table 3.
Multivariate analysis of odds of developing of acute respiratory distress syndrome
| Variable | Odds Ratioa | 95% Confidence Interval | p |
|---|---|---|---|
| Statin therapy | 0.69 | 0.51–0.92 | .01 |
| Age (per year) | 0.99 | 0.98–0.99 | <.01 |
| APACHE III (per point) | 1.01 | 1.01–1.02 | <.01 |
| Septic shock | 2.13 | 1.73–2.61 | <.01 |
| Pneumonia | 1.94 | 1.17–3.20 | .01 |
| Direct lung injury | 2.19 | 1.32–3.64 | <.01 |
| PRBC units transfused (per unit) | 1.04 | 1.02–1.06 | <.01 |
| Trauma | 1.44 | 0.93–2.22 | .10 |
| History of diabetes | 0.70 | 0.56–0.88 | <.01 |
| Serum total bilirubin (per mg/dL) | 1.04 | 1.01–1.07 | <.01 |
| Obesity (BMI >30) | 1.73 | 1.37–2.17 | <.01 |
| Morbid obesity (BMI >40) | 1.90 | 1.31–2.78 | <.01 |
APACHE, Acute Physiology and Chronic Health Evaluation; PRBC, packed red blood cell; BMI, body mass index.
Odds ratio for effect of each variable on risk of developing acute respiratory distress syndrome, as calculated in multivariate logistic regression model.
Table 4.
Multivariate analysis of odds of developing acute respiratory distress syndrome, matched analysis by propensity score for receipt of statin therapy
| Variable | Odds Ratioa | 95% Confidence Interval | p |
|---|---|---|---|
| Statin therapy | 0.80 | 0.58–1.11 | .19 |
| Age (per year) | 0.99 | 0.98–0.99 | .01 |
| APACHE III (per point) | 1.01 | 1.01–1.02 | <.01 |
| Septic shock | 2.29 | 1.84–2.84 | <.01 |
| Pneumonia | 2.09 | 1.22–3.58 | <.01 |
| Direct lung injury | 1.90 | 1.11–3.27 | .02 |
| PRBC units transfused (per unit) | 1.04 | 1.02–1.06 | <.01 |
| Trauma | 1.53 | 0.97–2.40 | .07 |
| History of diabetes | 0.69 | 0.54–0.90 | <.01 |
| Serum total bilirubin (per mg/dL) | 1.02 | 0.98–1.05 | .32 |
| Obesity (BMI >30) | 1.75 | 1.38–2.21 | <.01 |
| Morbid obesity (BMI >40) | 1.76 | 1.19–2.61 | <.01 |
APACHE, Acute Physiology and Chronic Health Evaluation; PRBC, packed red blood cell; BMI, body mass index.
Odds ratio for effect of each variable on risk of developing acute respiratory distress syndrome, as calculated in multivariate logistic regression model with matching by decile of propensity score.
We repeated the primary logistic regression analysis and included matching by decile of propensity score: 271 patients were excluded from matching because no patients in the lowest propensity score decile received statins. Of note, 102 of these patients (38%) had development of ARDS. Among the remaining 2,472 patients, 636 had development of ARDS (25.7%). In this analysis, the association between statin therapy and reduced development of ARDS was lost. Full results of the multivariable model are presented in Table 3. As described, we also conducted this analysis by including propensity score as a covariate in the logistic regression model instead of matching by propensity score decile to utilize all 2,743 patients in the analysis. The results were similar: statin therapy was again no longer associated with decreased odds of development of ARDS (OR 0.84; 95% CI 0.61–1.16; p = .29).
To determine whether there was a dose-related effect of statin therapy, analyses were again repeated after standardizing statin doses for different medications according to the method of Kendrach et al (24). This analysis yielded similar results as those of the analysis of statin therapy as an all-or-none phenomenon. Statin dose was associated with reduced odds of development of ARDS in the unmatched model (OR 0.91 per 10-unit increase in standardized statin dose; 95% CI 0.84–0.99; p = .03) but, as with the other analyses, the effect was lost after matching for propensity score decile (OR 0.96; 95% CI 0.88–1.05; p = .34).
Secondary Outcomes
At the 60-day time point, among the patients who had development of ARDS, 270 patients had died (37%). Median APACHE III score for ARDS patients was 76 (25%–75% interquartile range, 43–109). Baseline characteristics of the patients who had development of ARDS are shown in Table 5. Mortality was 43% (32 patients) in the group that received statins and 36% (238 patients) in the nonstatin group; however, the odds of mortality were not significantly different between groups (OR 1.32; 95% CI 0.81–2.15; p = .26). As with the primary outcome, we constructed a logistic regression model to determine the impact of statin therapy on 60-day mortality and conducted regression analysis both with and without matching according to propensity score for receiving statins. Receipt of statin therapy was not associated with altered odds of 60-day mortality in either the nonmatched or the matched analyses. Complete results of the multivariate analyses are shown in Tables 6 and 7.
Table 5.
Baseline characteristics of patients who developed acute respiratory distress syndrome (n = 738)
| Received Statin (n = 75) | Did Not Receive (n = 663) | p | |
|---|---|---|---|
| Age (mean ± SD) | 66.3 (±12.6) | 56.4 (±18.5) | <.01 |
| APACHE III score (mean ± SD) | 78.6 (±22.1) | 76.1 (±24.0) | .29 |
| Female gender | 38 (30%) | 228 (37%) | .13 |
| Caucasian race | 114 (90%) | 557 (91%) | .84 |
| Pneumonia | 90 (71%) | 438 (72%) | .97 |
| Sepsis w/o shock | 28 (22%) | 149 (24%) | .61 |
| Septic shock | 83 (66%) | 390 (64%) | .65 |
| Corticosteroid use | 9 (7%) | 58 (9%) | .40 |
| Diabetes history | 49 (39%) | 104 (17%) | <.01 |
| BMI | 28.8 (± 8.2) | 28.6 (± 8.9) | .80 |
| Obese (BMI >30) | 37 (29%) | 134 (22%) | .07 |
| Morbidly obese (BMI >40) | 7 (6%) | 50 (8%) | .32 |
| Cirrhossis | 1 (1%) | 43 (7%) | <.01 |
| Hepatic failure | 4 (3%) | 24 (4%) | .66 |
| Ethanol abuse | 9 (7%) | 110 (18%) | <.01 |
APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index.
Values represented as mean (± SD) for continuous variables and N (%) for categorical variables.
Table 6.
Multivariate analysis of odds of 60-day mortality (patients with acute respiratory distress syndrome only)
| Variable | Odds Ratioa | 95% Confidence Interval | p |
|---|---|---|---|
| Statin therapy | 1.21 | 0.69–2.13 | .50 |
| Age (per year) | 1.04 | 1.03–1.06 | <.01 |
| APACHE III (per point) | 1.03 | 1.02–1.04 | <.01 |
| Sepsis (without shock) | 1.43 | 0.92–2.22 | .11 |
| PRBC units transfused (per unit) | 1.06 | 1.03–1.10 | <.01 |
| Trauma | 0.17 | 1.03–1.10 | <.01 |
| History of liver cirrhosis | 3.02 | 1.28–7.09 | .01 |
| Corticosteroid therapy | 2.43 | 1.33–4.42 | <.01 |
| Serum total bilirubin (per mg/dL) | 1.11 | 1.04–1.18 | <.01 |
| Obesity (BMI >30) | 0.61 | 0.40–0.95 | .03 |
APACHE, Acute Physiology and Chronic Health Evaluation; PRBC, packed red blood cell; BMI, body mass index.
Odds ratio for effect of each variable on risk of death at 60 days of follow-up, as calculated in multivariate logistic regression model.
Table 7.
Multivariate analysis of odds of 60-day mortality, matched analysis by propensity score for receipt of statin therapy (patients with acute respiratory distress syndrome only)
| Variable | Odds Ratioa | 95% Confidence Interval | p |
|---|---|---|---|
| Statin therapy | 1.01 | 0.52–1.95 | .98 |
| Age (per year) | 1.04 | 1.03–1.06 | <.01 |
| APACHE III (per point) | 1.03 | 1.02–1.04 | <.01 |
| Sepsis (without shock) | 1.37 | 0.84–2.24 | .20 |
| PRBC units transfused (per unit) | 1.06 | 1.03–1.10 | <.01 |
| Trauma | 0.24 | 0.06–0.89 | .03 |
| History of liver cirrhosis | 2.86 | 1.07–7.64 | .04 |
| Corticosteroid therapy | 2.58 | 1.32–5.03 | <.01 |
| Serum total bilirubin (per mg/dL) | 1.07 | 1.00–1.15 | .04 |
| Obesity (BMI >30) | 0.62 | 0.38–1.02 | .06 |
APACHE, Acute Physiology and Chronic Health Evaluation; PRBC, packed red blood cell; BMI, body mass index.
Odds ratio for effect of each variable on risk of death at 60 days of follow-up, as calculated in multivariate logistic regression model with matching by decile propensity score.
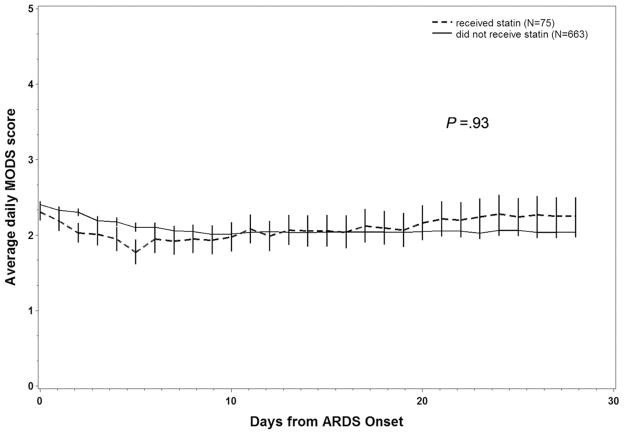
Average daily MODS scores are depicted in Figure 3. We compared MODS scores between patients who did and did not receive statins using a linear mixed-effects model (PROC MIXED in SAS 9.1.3), with age and admission APACHE III score included as fixed effects and daily MODS as repeated measures, assuming an unstructured covariance matrix. This model showed no difference between groups either with or without propensity score included in the model (p = .94 and p = .31, respectively).
Figure 3.
The 28-day average daily multiple organ dysfunction score (MODS) from patients who had development of acute respiratory distress syndrome (ARDS) compared between patients who received statins before intensive care unit admission and patients who did not.
We did not find a significant difference in number of ventilator-free days between patients who did and did not receive statins (median, 4.0; 25%–75% interquartile range, 0.0–11.0 vs. median 5.0; 25%–75% interquartile range, 0.0–12.0; p = .86). We performed an adjusted analysis of ventilator-free days using a generalized linear model including age and admission APACHE III score as covariates. This analysis also did not reveal a significant difference in ventilator-free days either by itself (p = .66) or when propensity score for receipt of statin was included as a covariate (p = .71).
Among all patients in the cohort, risk of death while in the ICU was not significantly different whether patients did or did not receive statins (OR 0.75; 95% CI 0.54–1.06; p = .10). We conducted logistic regression analysis as described, which showed that receipt of statins did not alter the risk of death while in the ICU among these patients, either in the multivariable analysis (OR 0.87; 95% CI 0.60–1.27; p = .46) or in the multivariable analysis using propensity score matching (OR 0.98; 95% CI 0.65–1.48; p = .94).
Prehospital Statin Use
We conducted additional sensitivity analyses to determine whether prehospital statin use was associated with altered risk of either the primary or the secondary outcomes; 607 of 2,743 patients were receiving statin therapy before hospital admission (22%), of whom 128 had development of ARDS (17%). Prehospital statin therapy was associated with reduced odds of development of ARDS (OR 0.65; 95% CI 0.52–0.81; p < .01). However, this association was no longer significant when adjusted for other clinical factors in a multivariate model (OR 0.85; 95% CI 0.66–1.09; p = .20) and remained nonsignificant when we applied propensity score matching(OR 0.98; 95% CI 0.73–1.32; p = .91).
Of the 128 patients who received pre-hospital statins and who had development of ARDS, 51 had died at 60 days (40%) and the odds of 60-day mortality were not significantly altered by statin therapy (OR 1.28; 95% CI 0.80–1.74; p = .41). We found similar results in the multivariate analysis (OR 1.25; 95% CI 0.78–1.99; p = .35) and propensity-matched multivariate analysis (OR 0.88; 95% CI 0.49–1.60; p = .68). We found no differences between groups in either daily MODS score or ventilator-free days (p = .93 and p = .70, respectively).
Continuation of Statins in Patients With ARDS
Among patients who had development of ARDS, a small proportion of patients continued to receive the drug after development of ARDS (62 of 738; 8%). Continuation of statin therapy after onset of ARDS was not associated with lower odds of mortality in unadjusted (p = .53), adjusted (p = .94), or adjusted and propensity-matched analyses (p = .87).
DISCUSSION
Our findings demonstrate that after patients were matched according to their propensity to receive statins, statin therapy was not associated with a decreased risk of development of ARDS among patients who presented to the ICU with ARDS risk factors. Among patients who did have development of ARDS, receipt of statin therapy was not associated with differences in 60-day or ICU mortality, ICU length of stay, ventilator-free days, or daily MODS scores.
Statin medications are commonly used for primary and secondary prevention of coronary heart disease. In addition to their primary effect on plasma lipoprotein levels, statins have pleiotropic anti-inflammatory and antioxidant effects, as well as effects on vascular endothelial function. These pleiotropic effects may be responsible for some of the benefits associated with statin therapy outside of the preventive setting, such as in acute myocardial infarction or in postoperative patients.
As such, statins have attracted much interest recently for a possible beneficial role in the treatment or prevention of critical illness, including sepsis and ARDS, as well as its less severe variant of acute lung injury (ALI). In animal models, statins demonstrate protective or stabilizing effects on the pulmonary vasculature in response to toxic injury (25–29). In animal models of acute lung injury, statins prevent, attenuate, or treat lung injury caused by various stimuli (9, 30–32). With regard to sepsis, a condition that shares many elements of pathophysiology with ALI/ARDS, similar findings in animal models have led to growing enthusiasm for their use as a potential treatment in that condition as well (33, 34).
This enthusiasm has sparked initiation of a number of studies, but thus far no large randomized controlled trials have been published. Observational studies in humans have yielded mixed results. With regard to the potential therapeutic applications of statins, the first question asked by our study is, “Among patients with risk factors for ARDS, did concurrent statin therapy prevent the development of ARDS?” Although preclinical evidence has been promising, to date human clinical studies have yielded only indirect insight into the answer. In epidemiologic and population-based studies, chronic statin use has been associated with decreased risk of critical illnesses, including sepsis, in several patient populations (10, 35–37), but these data pertain only partly to our question. More to our point, Almog et al (11) showed that among a group of 361 patients admitted to the hospital with infections, prehospital statin therapy was associated with decreased rates of severe sepsis and of ICU admission, lending credence to the possibility that statins might prevent the progression of critical illness among patients who were at risk. However, none of these studies specifically addressed the risk of development of ALI or ARDS.
Of slightly greater relevance to our question, a recent article by O’Neal et al (16) did study the prevalence of severe sepsis or ALI/ARDS in a cohort of 575 ICU patients and determined that the prevalence of these conditions was lower among patients who had been using statins, but that concurrent aspirin therapy might explain this effect. Whereas the cohort was smaller and differently comprised than our population (all ICU patients rather than patients specifically at risk for lung injury), and the definition of statin use was somewhat different, this study perhaps most directly addressed the question we pose. However, it and the other studies mentioned suffer from a shortcoming common to observational studies, that the decision to treat with statins was determined by forces external to the study. It is easy to envision the possibility that treatment with statins might be a surrogate for other factors involved with patient outcome and with general health, such as the presence of comorbidities, concurrent medications, socioeconomic status, greater attention to preventive healthcare maintenance, and others, thereby potentially introducing confounding by indication and selection bias.
The ideal approach for answering our research question would be to conduct a study wherein patients with direct risk factors were randomized to receive statin therapy or placebo on admission to determine whether the drug reduced their risk of development of ARDS. We have attempted to optimize our study design to replicate as many of these aspects as is possible. Our patient population was prospectively enrolled and well-characterized with regard to demographic and baseline data, and determination of ARDS risk factors, which was limited in some of the previous population-based studies. In addition to recording whether patients had been receiving statins as outpatients, as most studies have done, we recorded any administration of statin while in the hospital, thus better-establishing the potential temporal physiologic relationship between the drug and the outcome, thereby hopefully reducing the opportunity for confounding by other factors related to indications for outpatient statin use. Daily prospective screening for development of ARDS helps keep the outcome well-defined and less subject to dilution that may occur from including patients with less severe ALI. Finally, with regard to statin therapy, although we were unable to control which patients received the drugs, the use of propensity score matching helps limit confounding and bias introduced by nonrandom exposure to the drug and serves as an attempt to correct a between-group imbalance of covariates. Whereas we note that the potential for confounding is high (for example, as presented in Table 3, the differences in baseline variables between statin and nonstatin groups share much commonality with the factors associated with ARDS risk), propensity score matching serves to provide an unbiased or less biased estimation of treatment effects (38) in nonrandomized studies.
With regard to our findings, on the initial analysis we did in fact answer our research question in the affirmative–when patients with ARDS risk factors receive statins leading up to their ICU admission, they are less likely to have development of ARDS even after adjusting for multiple covariates. However, when propensity score matching is added to the analysis, the association disappears, suggesting that the reduced risk of ARDS is associated with another factor that tends to be related to statin use, whether it is measured or unmeasured. Given the body of evidence from preclinical studies, observational studies, and the nature of the association found in our study, there appears to be sufficient justification for a randomized controlled trial to settle the issues of potential confounding and bias.
Certainly, a preventive medication for ARDS is sorely needed. Four decades of research into pharmacologic therapy, primary anti-inflammatory therapy, for established disease has largely proven unfruitful (39). However, it is known that preventive strategies can be effective, including limiting mechanically ventilated tidal volumes and instituting more conservative blood product transfusion criteria (40). More recently, there have been efforts to define the timing of onset and to identify crucial early predictors of lung injury in the hopes of recognizing opportunities to institute preventive measures (41, 42). As an inexpensive commonly available class of medications, the statins could play a valuable role in prevention if proven to be effective.
Although studies specifically pertaining to treatment of lung injury are less common, of note a recent study by Kor et al suggested no benefit in outcomes in patients with ALI/ARDS when they received prehospital statins (14). The study design was similar to the analysis we performed for our secondary outcomes in a smaller cohort of 178 patients. Also of note, an epidemiologic study conducted among 196 ALI/ARDS patients in Ireland did not find a significant association between statin use and improved outcome (17). Our results are similar in that there was no indication of benefit in mortality, organ failure, or need for mechanical ventilation, although the rate of statin discontinuation after development of ARDS was high (with only 8% of patients being continued on statin after development of ARDS), making it difficult to draw conclusions. Among alternative possibilities, if statins had been continued in a higher proportion of patients, then a benefit might have been seen; therefore, this study is not ideally suited to study efficacy in this regard. Along similar lines, the withdrawal of statins might have led to deleterious effects in patients who had been receiving them previously.
Our study (and others) suffers from some important limitations. Statins in these cases were prescribed for other indications and, therefore, as discussed, there may be substantial bias or confounding associated with these analyses, inherent to any purely observational study. Despite our attempt to adjust via use of propensity score matching, residual bias or confounding could be present. Another important limitation is the dosing and administration of statins. In a controlled trial these regimens would be standardized, but we were limited to studying whatever medications and doses were prescribed by treating clinicians. Of note, however, a recent study has suggested that continuing statin therapy through ICU admission does not result in decreased inflammation or improved outcomes, although development of ARDS was not specifically studied (43). Additionally, the nature of the study (single academic medical center) leads to questions about external validity. Finally, given the potential for heterogeneity in ARDS pathophysiology according to the inciting risk factor, it is possible that statins have differential effects in some patients based on the underlying etiology. Such effects could explain the conflicting results seen in the literature or the overall neutral effect seen in our study.
CONCLUSIONS
In summary, our findings suggest that statins are not beneficial in the prevention of ARDS; however, to answer this question definitively, a randomized controlled trial is warranted. With regard to the utility of statins as a therapeutic agent in this disease, our study is consistent with others that do not show a substantial benefit. We look forward to the results from two ongoing randomized controlled trials to help answer this question.
Supplementary Material
Acknowledgments
Supported, in part, by NHBLI grants HL60710, HL67197, HL084060, and HL086667.
Drs. Bajwa, Christiani, and Gong receive grant support from the National Institutes of Health. Dr. Thompson is an investigator for the ARDS Network and Medical Director of the Clinical Coordination Center.
Footnotes
See also p. 1661.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://journals.lww.com/ccmjournal).
Dr. Malhotra has not disclosed any potential conflicts of interest.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: Adjustments and options. Am J Cardiol. 2005;96:53E–59E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Biasucci LM, Biasillo G, Stefanelli A. Inflammatory markers, cholesterol and statins: Pathophysiological role and clinical importance. Clin Chem Lab Med. 2010;48:1685–1691. doi: 10.1515/CCLM.2010.277. [DOI] [PubMed] [Google Scholar]
- 6.Bosma KJ, Taneja R, Lewis JF. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: Current and experimental approaches. Drugs. 2010;70:1255–1282. doi: 10.2165/10898570-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller HC, Hellwig K, Rosseau S, et al. Simvastatin attenuates ventilator-induced lung injury in mice. Crit Care. 2010;14:R143. doi: 10.1186/cc9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano K, Yamamoto S, Tomita K, et al. Successful treatment of acute lung injury with pitavastatin in septic mice: Potential role of glucocorticoid receptor expression in alveolar macrophages. J Pharmacol Exp Ther. 2011;336:381–390. doi: 10.1124/jpet.110.171462. [DOI] [PubMed] [Google Scholar]
- 9.Siempos II, Maniatis NA, Kopterides P, et al. Pretreatment with atorvastatin attenuates lung injury caused by high-stretch mechanical ventilation in an isolated rabbit lung model. Crit Care Med. 2010;38:1321–1328. doi: 10.1097/CCM.0b013e3181d9dad6. [DOI] [PubMed] [Google Scholar]
- 10.Almog Y, Novack V, Eisinger M, et al. The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med. 2007;35:372–378. doi: 10.1097/01.CCM.0000253397.42079.D5. [DOI] [PubMed] [Google Scholar]
- 11.Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers JD, Singanayagam A, Murray MP, et al. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med. 2008;121:1002–1007.e1. doi: 10.1016/j.amjmed.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Donnino MW, Cocchi MN, Howell M, et al. Statin therapy is associated with decreased mortality in patients with infection. Acad Emerg Med. 2009;16:230–234. doi: 10.1111/j.1553-2712.2009.00350.x. [DOI] [PubMed] [Google Scholar]
- 14.Kor DJ, Iscimen R, Yilmaz M, et al. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009;35:1039–1046. doi: 10.1007/s00134-009-1421-8. [DOI] [PubMed] [Google Scholar]
- 15.Shyamsundar M, McKeown ST, O’Kane CM, et al. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neal HR, Jr, Koyama T, Koehler EA, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acute lung injury and the acute respiratory distress syndrome in Ireland: A prospective audit of epidemiology and management. Crit Care. 2008;12:R30. doi: 10.1186/cc6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 19.Gong MN, Bajwa EK, Thompson BT, et al. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 21.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 22.Bernard GR, Doig G, Hudson L. Quantification of organ failure for clinical trials and clinical practice. Am J Resp Crit Care Med. 1995;151:A323. [Google Scholar]
- 23.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendrach MG, Kelly-Freeman M. Approximate equivalent rosuvastatin doses for temporary statin interchange programs. Ann Pharmacother. 2004;38:1286–1292. doi: 10.1345/aph.1D391. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson JR, Dudek SM, Birukov KG, et al. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004;30:662–70. doi: 10.1165/rcmb.2003-0267OC. [DOI] [PubMed] [Google Scholar]
- 27.Ozansoy G, Güven C, Ceylan A, et al. Effects of simvastatin treatment on oxidant/antioxidant state and ultrastructure of streptozotocin-diabetic rat lung. Cell Biochem Funct. 2005;23:421–6. doi: 10.1002/cbf.1168. [DOI] [PubMed] [Google Scholar]
- 28.Solovey A, Kollander R, Shet A, et al. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–6. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- 29.Naidu BV, Woolley SM, et al. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg. 2003;126:482–489. doi: 10.1016/s0022-5223(03)00699-8. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson JR, Barnard JW, Grigoryev DN, et al. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 31.Yao HW, Mao LG, Zhu JP. Protective effects of pravastatin in murine lipopolysaccharide-induced acute lung injury. Clin Exp Pharmacol Physiol. 2006;33:793–7. doi: 10.1111/j.1440-1681.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- 32.Shao H, Shen Y, Liu H, et al. Simvastatin suppresses lung inflammatory response in a rat cardiopulmonary bypass model. Ann Thorac Surg. 2007;84:2011–2018. doi: 10.1016/j.athoracsur.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Terblanche M, Almog Y, Rosenson RS, et al. Statins. Panacea for sepsis? Lancet Infect Dis. 2006;6:242–248. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 34.Rosch JW, Boyd AR, Hinojosa E, et al. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–635. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackam DG, Mamdani M, Li P, et al. Statins and sepsis in patients with cardiovascular disease: A population-based cohort analysis. Lancet. 2006;367:413–418. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R, Plantinga LC, Fink NE, et al. Statin use and sepsis events [corrected] in patients with chronic kidney disease. JAMA. 2007;297:1455–1464. doi: 10.1001/jama.297.13.1455. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen RW, Riis A, Kornum JB, et al. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med. 2008;168:2081–2087. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 39.Frank AJ, Thompson BT. Pharmacological treatments for acute respiratory distress syndrome. Curr Opin Crit Care. 2010;16:62–68. doi: 10.1097/MCC.0b013e328334b151. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: Protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35:1660–1666. doi: 10.1097/01.CCM.0000269037.66955.F0. [DOI] [PubMed] [Google Scholar]
- 41.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shari G, Kojicic M, Li G, et al. Timing to the onset of acute respiratory distress syndrome: A population-based study. Respir Care. 2011;56:576–582. doi: 10.4187/respcare.00901. [DOI] [PubMed] [Google Scholar]
- 43.Kruger PS, Harward ML, Jones MA, et al. Continuation of Statin Therapy in Patients with Presumed Infection: A Randomised Controlled Trial. Am J Respir Crit Care Med. 201l;183:774–781. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.