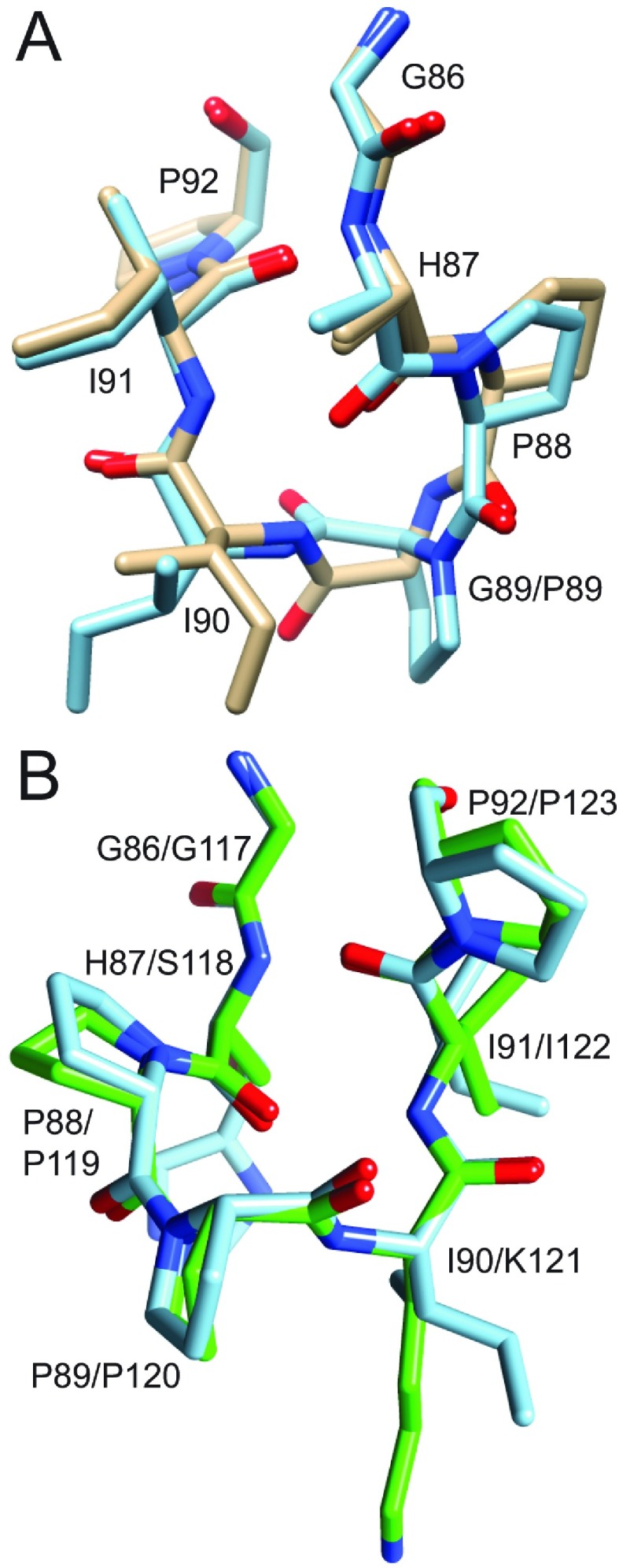
Figure 2. Superimposition of region surrounding the Pro88–Pro89 peptide bond in the crystal structure of the G89P variant (blue) as compared with the wild-type FKBP12 (gold) and to the homologous segment from the first FKBP domain of FKBP52 (green).
(A) In the comparison between the two FKBP12 structures, the His87 side chain is truncated at Cβ to facilitate visualization of the backbone conformations. The cis-peptide linkage in the G89P structure perturbs the backbone of residues 88–90, whereas the preceding and following residues superimpose fairly closely. (B) As viewed from the opposite face of the loop, the structural changes induced by the cis-peptide linkage in the G89P variant yields a backbone conformation that closely follows that of the first FKBP domain of FKBP52 (PDB code 1N1A).