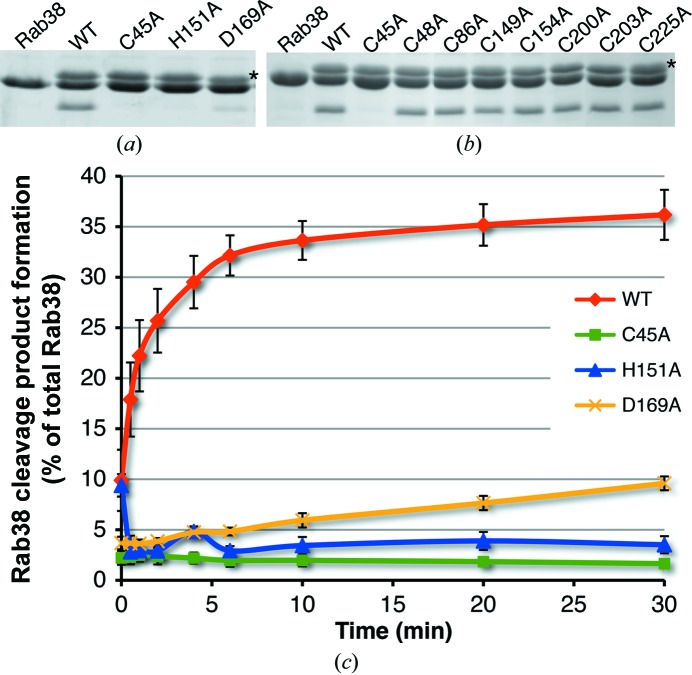
Figure 4.
(a) Mutations (C45A, H151A and D169A) of the catalytic triad of full-length GtgE greatly reduce the enzyme activity in vitro. GtgE runs slightly larger than Rab38, and the row of bands corresponding to GtgE is indicated with an asterisk to the right of the panel. (b) The Cys-to-Ala mutation activity profile for GtgE. Each cysteine mutant was tested for cleavage activity against Rab38 as described. Of all eight cysteine mutants, only Cys45Ala shows a loss of function, indicating that it is the active cysteine of GtgE. The top row of bands corresponds to GtgE and is indicated with an asterisk to the right of the panel. (c) The catalytic triad residues (Cys45, His151 and Asp169) were mutated to alanines and their ability to cleave Rab38 is charted as a percentage of the total Rab38 cleaved over time in minutes. The standard error of the mean is indicated with black bars for each time point.