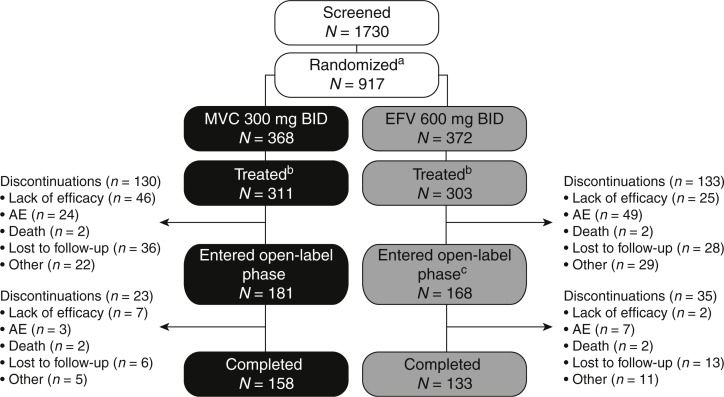
Fig. 1.
Study disposition.
aOf the 917 randomized patients, 177 patients were included in the MVC q.d. arm, which was subsequently discontinued; bpatients with confirmed R5 HIV-1 treated in the double-blind phase; ctwo patients who completed double-blind EFV treatment did not enter the open-label phase. AE, adverse event; b.i.d., twice daily; EFV, efavirenz; HIV, human immunodeficiency virus; MVC, maraviroc; q.d., once daily; R5, chemokine co-receptor type 5.