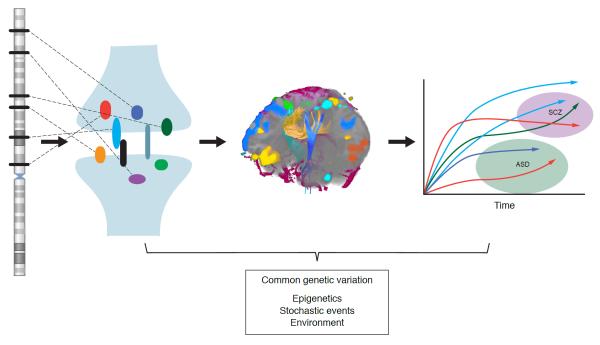
Figure 2. Convergent rare risks and common modulators leading to divergent neuropsychiatric disorders.
The figure illustrates one explanatory model for the combination of specific rare variations, carrying large effects for ASD, with common functional variants modulating the risk for clinically distinct phenomenon. The ideogram on the left represents the human genome and the black bars, the high degree of locus heterogeneity underlying ASD; the dotted lines link divergent loci to rare mutations that disrupt protein function and/or structure and sub-serve coherent molecular pathways. Here this is illustrated as multiple mutations in synaptic molecules, though as noted in the text, it is likely that a variety of distinct neural-developmental processes are altered by ASD-related mutations. In this model, alterations to basic cellular and molecular processes are expressed through increasingly complex layers of organization, represented in the figure by the diffusion tensor imaging data showing long-range neural pathways. Moreover, the manifestations of these altered molecular and cellular processes play out across development and are influenced by a variety of factors, including, importantly, common functional genetic variation (most likely regulatory in nature), as well as stochastic effects, environmental inputs and epigenetic phenomenon (shown in the text box at the bottom of the figure). The graph on the far right further illustrates that particular genetic variations may launch and simultaneously influence diverse developmental trajectories (lines of similar colors reflect rare mutations in the same CNV or gene) that may ultimately correspond to a range of distinct clinical phenomenon (in this example ASD and schizophrenia (Scz)) or to typical development.