Abstract
It was demonstrated that combination antiretroviral therapy (cART) reduces the HIV-1 viral load (VL) in the blood and the seminal compartment. Some studies have reported that the seminal HIV-1 VL is undetectable in individuals with an undetectable blood plasma viral load (bpVL) under cART. However, some recent studies have demonstrated that seminal HIV-1 RNA may still be detected, and potentially infectious, even in the case of an undetectable bpVL. The aim of this retrospective study was to determine the detection rate of a seminal VL and whether shedding could be intermittent over a very short time.
From January 2006 to December 2011, 88 HIV-1 infected men, enrolled in an Assisted Reproduction program, provided 306 semen samples, corresponding to 177 frozen sperm samples (two samples delivered at a one-hour interval (n = 129) or one sample (n = 48)). All enrolled men were under cART, with an undetectable bpVL for more than 6 months. HIV-1 RNA was quantified in seminal plasma using a Roche COBAS Ampliprep COBAS TaqMan HIV-1 test.
Seminal HIV-1 RNA was detected in 23 samples (7.5%) from 17 patients (19.3%). This detection rate was stable over years. With regards to the freezing of two samples delivered at a one-hour interval, the proportion of discordance between the first and second samples was 9.3% (12/129).
Our results confirm the intermittent shedding of HIV-1 in semen. While this finding has been shown by studies examining longer time intervals, to our knowledge, this has never been demonstrated over such a short time interval.
Introduction
HIV-1 remains wide spread throughout the population. There were approximately 6,088 new infections, resulting in a total of 150,000 infected people in France in 2011 [1]. Nearly 60% were acquired through heterosexual intercourse, despite the low efficiency of HIV-1 transmission via the sexual route (the risk of male-to-female intra-vaginal HIV-1 transmission is estimated to be approximately 8 per 1000 sexual acts) [2].
In this context, Combination Antiretroviral Therapy (cART) has remarkably improved the quality of life and the life expectancy of the HIV-1 infected population. It has also allowed serodifferent couples to consider natural procreation. Assisted Reproduction programs (AR) were established in 1992 to offer HIV-1 infected men the means to safely procreate [3]. Using AR, no seroconversion has been reported in serodifferent couples in which the men are HIV-1 infected [4].
It was demonstrated that cART reduces the HIV-1 viral load in the blood and the seminal compartment [5], [6]. Some studies have reported that the risk of HIV-1 transmission is minimal via sexual intercourse for HIV-1 infected individuals who are successfully treated with cART [7], [8], have no other sexually transmitted diseases and have had an undetectable HIV-1 plasma viral load for more than 6 months [9], [10]. However, recent studies have demonstrated that HIV-1 RNA may still be detectable and potentially infectious in semen, even if undetectable in blood plasma [11]–[15] (Table 1). The aims of this retrospective study were the following: (i) to evaluate the detection rate of a seminal viral load in a cohort of HIV-1 infected men under cART who had undetectable blood plasma HIV-1 RNA viral loads (bpVLs) for more than 6 months and (ii) to determine if HIV-1 shedding in semen could be intermittent over a very short time. We also investigated factors associated with the detection of HIV-1 RNA in seminal plasma.
Table 1. Summary of the different studies with seminal plasma sample analysis of HIV-1 patients under combination antiretroviral therapy and with an undetectable blood plasma viral load.
| Numbers | HIV-1 RNA detection thresholds (copies/ml) | ||||||||
| Studies | Studied period | Patients | SPS | Delay between bpVL and spVL | bpVL | spVL | Prevalence of detectable spVL | Prevalence of patients with at least one detectable spVL | cART regimen of patients with at least one detectable spVL** |
| Vernazza et al. [6] | N/A | 114 | N/A | synchronized | 400 | 400 | N/A | 1.8% (2/114) | Grp 2: 1/2, Grp 3: 1/2 |
| Bujan et al. [17] | 04/1998 - 01/2001 | N/A | N/A | N/A | 20 | 100 | 7.9% (N/A) | N/A | N/A |
| Marcelin et al. [12] | 01/2002 - 01/2008 | 140* | 232 | synchronized | 40 | N/A | 3% (7/232) | 5% (7/140*) | Grp 1: 14.3% (1/7), Grp 2: 57.1% (4/7), Grp 3: 28.6% (2/7) |
| Sheth et al. [11] | N/A | 25 | 116 | synchronized | 50 | 300 | 16.4% (19/116) | 48% (12/25) | Grp 1: 58.3% (7/12); Grp2: 42.7% (5/12) |
| [11] long-term follow-up | N/A | 13 | 13 | synchronized | 50 | 30 | 31% (4/13) | 31% (4/13) | Grp1: 2/4, Grp 2 : 2/4 |
| Halfon et al. [13] | 10/2001 - 03/2009 | 224 | 263 | 28 days (median) | 40 | 40 | 3.8% (10/263) | 4% (9/224) | Grp 1: 0% (0/10), Grp 2: 50% (5/10), Grp 3: 50% (5/10) |
| Dulioust et al. [10] | 01/2002 - 12/2009 | 455 | N/A | up to 2 months | 50 | 100 | N/A | Overall: 3.7% (17/455), 2002*: 15% (6/38), 2003*: 10% (6/60), 2004*: 6% (4/65), 2005*: 1.5% (1/80), 2006–2009 : 0% (0/212) | N/A |
| Lambert-Niclot et al. [14] | 01/2002 - 06/2011 | 304 | 628 | N/A | 20 or 40 | 100 or 200 | N/A | Overall: 6.6% (20/304), 2002: 0% (0/16), 2003*: 3% (1/35), 2004*: 4% (2/30), 2005*: 3% (1/37), 2006*: 4% (1/25), 2007*: 7% (2/37), 2008*: 5% (3/60), 2009* : 6% (4/65), 2010*: 5.5% (3/55), 2011*: 11% (3/27) | Grp 1: 10% (2/20), Grp 2: 70% (14/20), Grp 3: 20% (4/20) |
| Our study | 01/2006 - 12/2011 | 88 | 306 | up to 6 months | 50 | 200 | Overall: 7.5% (23/306) | Overall: 19.3% (17/88) | Grp 1: 11.8% (2/17), Grp 2: 82.4% (14/17), Grp 3: 5.9% (1/17) |
N/A, not available; cART, combination antiretroviral therapy; SPS, seminal plasma sample; bpVL, blood plasma viral load; spVL, seminal plasma viral load; Grp, group.
* Estimated results based on figures and numbers in published studies.
** Group 1: 1 non-nucleosidic reverse transciptase inhibitor+2 nucleosidic reverse transciptase inhibitor, Group 2: 1 protease inhibitor+2 nucleosidic reverse transciptase inhibitor, Group 3: other combination.
Materials and Methods
We retrospectively analysed data from 88 HIV-1 infected men who enrolled in the AR program of Bichat – Claude Bernard Hospital (Paris, France), from January 2006 to December 2011. The men provided 306 semen samples, corresponding to 177 frozen sperm samples (FSs) (mean 2.0±1.4 FSs per patient). Forty-eight of the FSs were obtained from one initial sample, and 129 were obtained from two initial samples provided at a one-hour interval. The mean number of samples per patient was 3.5±2.3, with each patient providing 1 to 14 samples.
All men were under cART, with a bpVL <50 copies/ml for more than 6 months. cART regimens included two nucleosidic reverse transcriptase inhibitors (NRTIs) plus a non-NRTI or a protease inhibitor (PI) and other antiretroviral combinations. In French AR programmes, before inclusion, each couple must be validated by a multidisciplinary committee that confirms the inclusion criteria, including treatment adherence and follow-up for HIV-1 infected patients [16]. The final measure of patient blood plasma HIV-1 RNA levels was performed between 6 months before the day of FS. Patients were also asked about their treatment adherence over the past 6 months before each FS. Socio-demographic, clinical and biological data were collected at the beginning of the AR programme and included the following: age, geographic origin, sperm characteristics (semen volume, sperm concentration, mobility and vitality, seminal leukocyte counts), CD4 cell count nadir, route of HIV transmission, HBV or HCV co-infection status and cART regimen.
Semen samples were obtained by masturbation after 2–7 days of sexual abstinence. Each patient provided, if possible, 2 semen samples within a one-hour interval, according to the routine protocol in our AR centre for FS. After liquefaction, semen samples were separately centrifuged through a two-layer discontinuous gradient. After processing, the seminal plasma sample (SPS) (supernatant) was separated and transferred to the virology department for seminal plasma HIV-1 viral load (spVL) quantification using a Roche COBAS Ampliprep COBAS TaqMan HIV-1 test (Roche Diagnostics, Meylan, France) with a detection threshold of 200 copies/ml [17].
Statistical analysis was performed using SAS 9.2. The Fisher exact test and the Wilcoxon test were used, and a p-value<0.05 considered statistically significant. Our unit of analysis was the patients with at least one detectable seminal HIV-1 RNA measurement because most of the patients' characteristics used in the analysis were not repeated over time.
All samples were collected in our AR department, and all collected data were anonymised before processing. As this study included only patients referred from routine follow-up, no supplemental blood punctures or sperm retrieval were performed. Obtaining written consent is not required, as stipulated by the French Government Rule (“Loi informatique et Liberté” – Chapter IX, Article 57). The study protocol and lack of patient consent was approved by the French national commission of information and individual liberties (CNIL).
Results
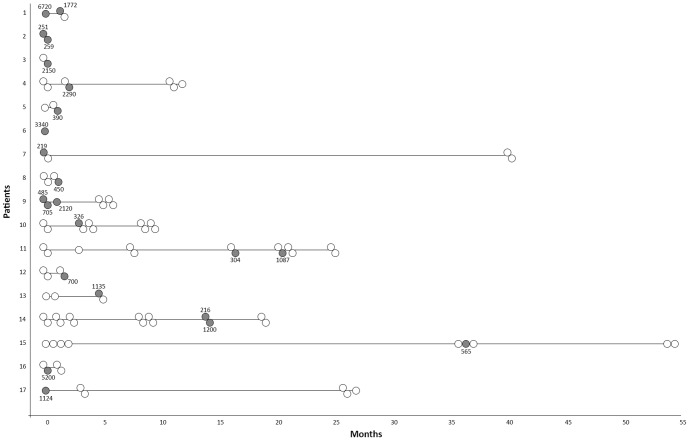
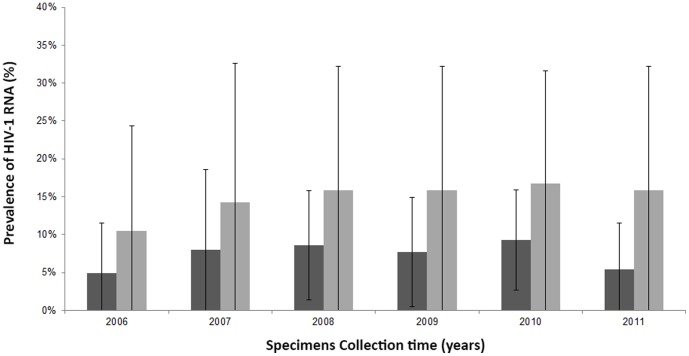
HIV RNA was detected in at least one SPS for 17 patients (19.3%) during the study period, corresponding to 23 SPSs (7.5%) (Table 2). SpVL ranged between 200 and 300 copies/ml in 4 SPSs, between 300 and 1000 copies/ml in 8, and between 1000 and 3000 copies/ml in 8; the spVL was above 3000 copies/ml in 3 SPSs (median 705 copies/ml) (Figure 1). The detection rate of spVL and the rate of patients with at least one SPS with a detectable viral load were stable over time (Figure 2, Table 1).
Table 2. Characteristics of the 17 patients with at least 1 detectable HIV-1 seminal plasma viral load.
| Patient | Age (years) | Co-infection | HIV transmission route | CD4 cell count (cells/µl) | Antiretroviral regimen |
| 1 | 31–35 | / | transfusion | 831 | TVF, ABC, ATZ, RTV |
| 2 | 31–35 | / | heterosexual | 302 | TVF, FTC, LPV, RTV |
| 3 | 41–45 | / | homosexual | 624 | TVF, DDI, ATZ, RTV |
| 4 | 41–45 | HCV | heterosexual | 489 | TVF, FTC, EFV |
| 5 | 36–40 | / | heterosexual | 417 | TVF, FTC, EFV, LPV, RTV |
| 6 | 31–35 | / | heterosexual | 294 | ZDV, 3TC, ATZ, RTV |
| 7 | 36–40 | HBV | undetermined | 419 | TVF, FTC, ATZ, RTV |
| 8 | 40–45 | / | heterosexual | 545 | TVF, FTC, ATZ, RTV |
| 9 | 46–50 | HCV | drug addiction | 830 | DDI, 3TC, LPV, RTV |
| 10 | 31–35 | / | drug addiction | 302 | TVF, FTC, LPV, RTV |
| 11 | 36–40 | / | heterosexual | 906 | ZDV, 3TC, FPV, RTV |
| 12 | 31–35 | / | heterosexual | 429 | ZDV, 3TC, LPV, RTV |
| 13 | 41–45 | / | heterosexual | 1676 | ZDV, 3TC, ATZ, RTV |
| 14 | 31–35 | / | heterosexual | 648 | TVF, FTC, LPV, RTV |
| 15 | 36–40 | / | heterosexual | 706 | ZDV, 3TC, LPV, RTV |
| 16 | 41–45 | / | heterosexual | 401 | TVF, FTC, EFV |
| 17 | 31–35 | / | undetermined | 609 | TVF, FTC, ATZ, RTV |
3TC, lamivudine; ATZ, atazanavir; ABC, abacavir; DDI, didanosine; EFV, efivarenz; FTC, emtricitabine; LPV, lopinavir; RTV, ritonavir; TVF, tenofovir; ZDV, zidovudine.
Figure 1. Pattern of HIV-1 shedding in the semen of 17 patients with at least one detectable seminal plasma viral load.
Each horizontal line represents the data for one subject. Seminal plasma viral loads are represented by circles coloured as follows: white, undetectable; dark grey, detectable. The viral loads (copies/ml) of detectable samples are annotated near the dark grey circles. Two juxtaposed circles represent samples provided at a one-hour interval.
Figure 2. Prevalence of detectable seminal plasma samples (dark grey) and of men with at least one detectable spVL (light grey) from 2006 to 2011.
Bars represent the 95% confidence intervals.
Of the 129 FSs in which two samples were provided at a one-hour interval, the spVL results in 117 (90.7%) were concordant for the first and the second SPSs, and 12 (9.3%) were discordant. In 8 cases, a spVL was undetectable in the first SPS and detectable in the second. In 4 cases, a spVL was first detectable and later undetectable. There was no significant association with the timing of the specimen. In the 12 discordant cases, the median spVL was 918 copies/ml, with a range between 200 and 500 copies/ml in 5, and between 500 and 1000 copies/ml in one; the spVL was over 1000 copies/ml in 6 cases (Figure 1).
No significant correlation between a detectable spVL and the collected data was demonstrated. More men treated with 2 NRTIs+1 PI had at least one detectable spVL (28.6%; 14/49) compared to those treated with 2 NRTIS+1 NNRTI (7.7%; 2/26) or other cART regimens (7.7%; 1/13); however, this association was not statistically significant (p = 0.054).
Discussion
We show that HIV-1 RNA levels were above 200 copies/ml in 7.5% (23/306) of the collected seminal plasma samples from patients administered cART and with undetectable blood plasma HIV-1 RNA for at least 6 months. This rate was stable during the study period. This result is consistent with some previous studies [13], [14], different from others, which reported that the spVLs was undetectable in all samples collected after 2005 [10]. In our study, the percentage of patients with detectable HIV-1 RNA in semen (19.3%) was higher than the percentages previously reported, despite a similar or lower detection threshold (Table 2). One explanation could be the longer period (up to 6 months) between the last available blood plasma sample used for the HIV-1 RNA quantification compared with the spVL. Nevertheless, the overall detection rate of a spVL was not correlated with this delay (mean delay of 2.8±1.7 months for samples with undetectable HIV-1 RNA versus 2.6±1.7 in detectable samples).
The intermittent seminal shedding of HIV-1 RNA observed in samples collected at a one-hour interval is in agreement with that reported for longer intervals [18], [19]. Of note, this intermittence has not yet been demonstrated for such timing (one-hour intervals).
The tendency towards a higher risk of a detectable spVL in patients given the PI-containing cART regimen compared to a regimen containing an NNRTI might be explained by the poor diffusion of most PIs in the male genital tract [20]. This treatment regimen is given to 50–70% of patients with at least one sperm sample with a detectable viral load in the reported series, whereas studies describing patients with permanently undetectable HIV-1 RNA in semen occasionally report treatment regimen [11]–[14] (Table 1).
In conclusion, we show that intermittent shedding of HIV-1 RNA in the semen of patients given efficient cART could occur within a one-hour interval. This timing should not be considered to place individuals at greater risk for HIV transmission than previously reported. Indeed, the probability of HIV transmission from a sperm viral load of 1,000 copies/ml has been estimated to be 3 per 10,000 episodes according to a probabilistic empiric model [21]. The risk of heterosexual transmission of HIV-1 has been reported as non-significant (0 per 100 person-years, 95% CI = 0–0.05) when full virologic suppression on cART is achieved in the infected partner [8] and when pre-exposure prophylaxis is used for uninfected women for unprotected sexual intercourse during fertile days [22]. Nevertheless, the 7.5% detection rate of HIV-1 RNA in seminal plasma samples from the same successfully treated patients and the nearly 20% proportion of those HIV-1 infected men with at least one semen sample with a detectable viral load, which was stable over a five-year period, should balance messages on the individual risk of HIV transmission through unprotected sex as an exclusive preventive strategy in serodifferent couples with procreation desires.
Supporting Information
Loi informatique et Liberté – Chapter IX, Article 57. French government rule on treatment of personal data in medical studies.
(DOCX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Cazein F, Le Strat Y, Le Vu S, Pillonel J, Lot F, et al. (2012) HIV testing in France, 2003–2011. BEH 46–47: 529–533. [Google Scholar]
- 2. Boily M, Baggaley R, Wang L, Masse B, White R, et al. (2009) Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis 9: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Semprini A, Levi-Setti P, Bozzo M, Ravizza M, Taglioretti A, et al. (1992) Insemination of HIV-negative women with processed semen of HIV-positive partners. Lancet 340: 1317–1319. [DOI] [PubMed] [Google Scholar]
- 4. Bujan L, Hollander L, Coudert M, Gilling-Smith C, Vucetich A, et al. (2007) Safety and efficacy of sperm washing in HIV-1-serodiscordant couples where the male is infected: results from the European CREAThE network. AIDS 21: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 5. Leruez-Ville M, Dulioust E, Costagliola D, Salmon D, Tachet A, et al. (2002) Decrease in HIV-1 seminal shedding in men receiving highly active antiretroviral therapy: an 18 month longitudinal study (ANRS EP012). AIDS 16: 486–488. [DOI] [PubMed] [Google Scholar]
- 6. Vernazza P, Troiani L, Flepp M, Cone R, Schock J, et al. (2000) Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS 14: 117–121. [DOI] [PubMed] [Google Scholar]
- 7. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 Infection with Early Antiretroviral Therapy. NEJM 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loutfy R, Wu W, Letchuman M, Bondy L, Antoniou T, et al. (2013) Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PloS one 8: e55747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vernazza P, Hirschel B, Bernasconi E, Flepp M (2008) HIV seropositive persons without sexually transmitted diseases under fully suppressive antiretroviral treatment do not sexually transmit HIV. BMS 89: 165–169. [Google Scholar]
- 10. Dulioust E, Leruez-Ville M, Guibert J, Fubini A, Jegou D, et al. (2010) No detection of HIV 1-RNA in semen of men on efficient HAART in the past 4 years of a 2002–2009 survey. AIDS 24: 1595–1598. [DOI] [PubMed] [Google Scholar]
- 11. Sheth P, Kovacs C, Kemal K, Jones R, Raboud J, et al. (2009) Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS 23: 2050–2054. [DOI] [PubMed] [Google Scholar]
- 12. Marcelin A, Tubiana R, Lambert-Niclot S, Lefebvre G, Dominguez S, et al. (2008) Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS 22: 1677–1679. [DOI] [PubMed] [Google Scholar]
- 13. Halfon P, Giorgetti C, Khiri H, Penaranda G, Terriou P, et al. (2010) Semen may harbor HIV despite effective HAART: another piece in the puzzle. PloS one 5: e10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambert-Niclot S, Tubiana R, Beaudoux C, Lefebvre G, Caby F, et al. (2012) Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002–2011 survey. AIDS 26: 971–975. [DOI] [PubMed] [Google Scholar]
- 15. Pasquier C, Saune K, Raymond S, Moinard N, Daudin M, et al. (2009) Determining seminal plasma human immunodeficiency virus type 1 load in the context of efficient highly active antiretroviral therapy. J Clin Microbiol 47: 2883–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachelot-Narquin R (2010) Arrêté du 3 août 2010 modifiant l'arrêté du 11 avril 2008 relatif aux règles de bonnes pratiques cliniques et biologiques d'assistance médicale à la procréation. Journal Officiel de la République Française n°0211. 16520 p.
- 17. Pasquier C, Anderson D, Andreutti-Zaugg C, Baume-Berkenbosch R, Damond F, et al. (2006) Multicenter quality control of the detection of HIV-1 genome in semen before medically assisted procreation. J Med Virol 78: 877–882. [DOI] [PubMed] [Google Scholar]
- 18. Bujan L, Daudin M, Matsuda T, Righi L, Thauvin L, et al. (2004) Factors of intermittent HIV-1 excretion in semen and efficiency of sperm processing in obtaining spermatozoa without HIV-1 genomes. AIDS 18: 757–766. [DOI] [PubMed] [Google Scholar]
- 19. Gupta P, Leroux CF, Patterson B, Kingsley L, Rinaldo C, et al. (2000) Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral quasi species between blood and semen. J Infect Dis 182: 79–87. [DOI] [PubMed] [Google Scholar]
- 20. Taylor S, Pereira A (2001) Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect 77: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chakraborty H, Sen P, Helms R, Vernazza P, Fiscus S, et al. (2001) Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15: 621–627. [DOI] [PubMed] [Google Scholar]
- 22. Vernazza P, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A (2011) Pre exposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 25 (16) 2005–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loi informatique et Liberté – Chapter IX, Article 57. French government rule on treatment of personal data in medical studies.
(DOCX)