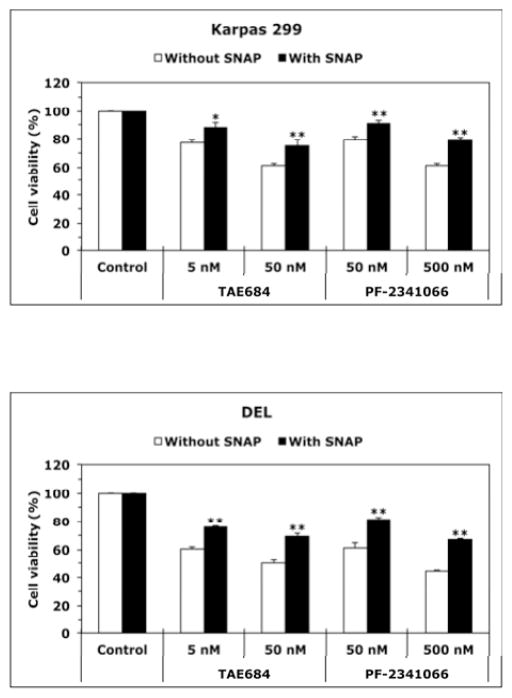
Figure 2. Inhibition of iNOS/NO signaling decreases the viability, adhesion, and migration of NPM-ALK+ T-cell lymphoma cells.
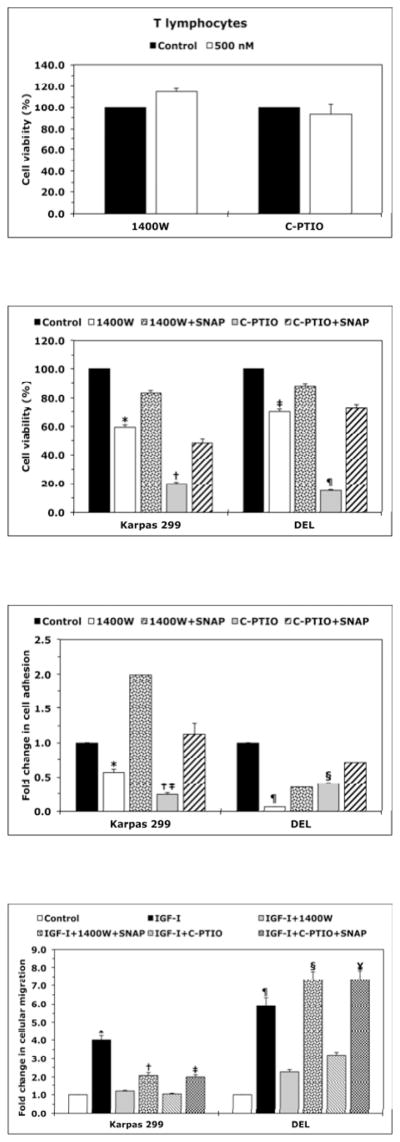
(A) The iNOS inhibitor 1400W (500 nM) and the NO scavenger C-PTIO (500 nM) did not change viability of T lymphocytes at 48 h after treatment; (B) In contrast, 1400W and C-PTIO decreased the viability of Karpas 299 and DEL cells at 24 h after treatment, and the NO donor SNAP (500 nM) attenuated these effects (*: p < 0.001 vs. control and 1400W+SNAP, †: p < 0.001 vs. control and C- PTIO+SNAP; ‡: p < 0.0001 vs. control and 1400W+SNAP; ¶: p < 0.0001 vs. control and C- PTIO+SNAP); (C) Inhibition of iNOS/NO signaling reduced the adhesion of Karpas 299 and DEL cells to endothelial cells and SNAP reversed these effects (*: p < 0.001 vs. control and 1400W+SNAP, †: p < 0.0001 vs. control, p < 0.01 vs. C-PTIO+SNAP, ‡: p < 0.00001 vs. control and 1400W+SNAP, ¶: p < 0.00001 vs. control and C-PTIO+SNAP); (D) IGF-I stimulated the migration of Karpas 299 and DEL cells, and 1400W and C-PTIO inhibited the effects of IGF-I. Additional treatment with SNAP reversed the effects of 1400W and C-PTIO (*: p < 0.001 vs. control, IGF-I+1400W, and IGF-I+C-PTIO, †: p < 0.01 vs. control and IGF-I+1400W, ‡: p < 0.001vs. control and IGF-I+C-PTIO, ¶: p < 0.001 vs. control and IGF-I+1400W and p < 0.01 vs. IGF-I+C- PTIO, §: p: < 0.001 vs. control and IGF-I+1400W, ¥: p < 0.001 vs. control and p < 0.01 vs. IGF-I+C- PTIO); The ALK inhibitors TAE684 or PF-2341066 induced a concentration-dependent decrease in the viability of Karpas 299 (E) and DEL (F) cells at 24 h. These effects were significantly diminished when cells were simultaneously treated with the ALK inhibitor and SNAP (*: p < 0.001, **: p < 0.0001 for cells treated with the ALK inhibitors and SNAP vs. cells treated with ALK inhibitors alone). Data shown are the means ± SE of 3 independent experiments.