Abstract
Background
The thumb, or digit 1, is not a typical digit. In addition to its unusual mobility and function, its formation is also unusual. It is the last digit to form and the most commonly targeted when limb development is disrupted. The thumb domain is defined by the overlapping expression of HOXA13, TBX5, GLI3R, and HOXD13 and, importantly, by an absence of other distal HOXD transcription factors. This brief review, combining developmental biology and clinical genetics, discusses the current understanding of how the thumb domain is established.
Introduction
The basic layout of the upper limb and hand is largely conserved across tetrapods with preservation of the radial to ulnar metacarpal/phalangeal pattern (2,3,3,3,3) from reptiles to primates. The two-phalangeal thumb is a distinctive structural feature. Its shape, position, and structure can be used to distinguish species, whether a hand is right or left, and even whether a hand is from a male or female. Although the thumb has features common to all digits, its differences are striking, species-specific, and demonstrate independence in its developmental programming. The description here combines clinical genetics and developmental biology from animal models. Although animal models are inadequate to fully characterize the human condition, they do provide insight into the processes and initial pathways involved.
The First Is Last
The autopod or handplate is the last segment of the upper limb to differentiate. In humans, toward the end of the fifth week of development, the distal end of the limb bud begins to remodel, expanding and flattening to form the autopod or handplate [21]. The thumb is also the last of the digits on the hand to form [9]. This terminal position in development appears to place the thumb at great risk of developmental disruption; over 1100 syndromes in the Online Mendelian Inheritance in Man (OMIM) database have hypoplastic thumbs as a syndromic feature (search done on November 1, 2012). However, this sensitivity to disruption may also reflect a capacity for rapid morpohologic modification, ie, punctuated equilibrium, and account for the marked differences in species-related thumb function [7]. The number of digits formed is also related to limb bud width [16]. Thus, disruptions that nonspecifically decrease limb mass or width may preferentially target the thumb. This has been experimentally demonstrated in reptiles using cytosine-arabinofuranoside, a cytotoxic chemotherapy drug that targets proliferating cells [22, 23]. In these animals with reduction of limb volume, the thumb was the first digit to be lost.
Cell Death Highlights the Presumptive Thumb
As the handplate forms, targeted regions of the limb begin to undergo programmed cell death. At the anterior or radial border of the handplate, at a stage corresponding to Carnegie stage 16 (day 37, postovulation) in humans, a cluster of cells in the mouse forelimb termed foyer préaxiale primaire (fpp) undergoes programmed cell death or apoptosis just proximal to the region of the presumptive thumb [8, 18] (Fig. 1). In mice with preaxial polydactyly, apoptosis in the fpp is absent [14, 18].
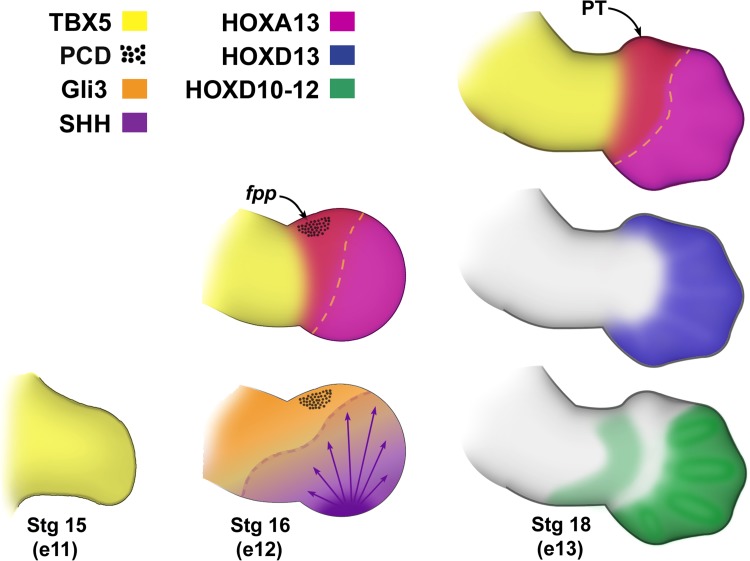
Fig. 1.
Defining the thumb. The thumb domain is defined by the overlapping expression of HOXA13, TBX5, GLI3R, and HOXD13 and, importantly, an absence of other HOXD transcription factors. Before handplate formation (Carnegie Stg 15), TBX5 is expressed throughout the limb bud; however, as the handplate forms (Carnegie Stg 16), TBX5 expression extends into the presumptive thumb domain (boundary highlighted by yellow dashed lines), HOXA13 is induced, and a region adjacent to the presumptive thumb (PT), the foyer préaxiale primaire (fpp), undergoes programmed cell death (PCD). Processing of GLI3 by SHH sets up an AP gradient of GLI3 repressor (GLI3R) to GLI3 activator, respectively. SHH also regulates the patterning of the distal HOXD transcription factors (HOXD11–13) (Carnegie Stg 18) that physically interact with GLI3 to refine digit identity (the SHH-dependent boundary is highlighted by a purple dashed line). HOXD 10–12 have overlapping expression domains in presumptive digits 2 to 5 but are restricted from the thumb domain. In contrast, HOXD13 is expressed in all of the digit domains including the presumptive thumb. Note the corresponding embryonic days (e 11–13) of the mouse limb from whence these data have been derived.
Although a great deal is known about the apoptotic pathway, the molecular basis for triggering this targeted cell death in limbs is less well characterized. The Gli-Krupple zinc finger transcription factor 3 (GLI3) is expressed throughout the limb bud. GLI3 is a transcription factor of the sonic hedgehog (SHH) pathway that can act as both an activator and a repressor [34]. SHH emanating from the zone of polarizing activity (ZPA) in the posterior or ulnar aspect of the limb maintains the GLI3 transcription factor as a full-length activator and establishes a gradient along the anteroposterior (AP), or radioulnar, axis [4, 17, 34]. Beyond the influence of SHH signaling, ie, the presumptive thumb region, GLI3 is processed into a repressor (GLI3R) that enhances apoptosis and inhibits digit formation [17, 30, 34]. Progressive loss of SHH signaling increases the expression domain of GLI3R, reduces limb width, and results in a loss of digits (note, although the addition of “ulnar aspect” was in response to a reviewer query, in further review, loss of the thumb can also occur with reduced limb width from Shh and thus it is better to end this sentence simply as an unqualified “loss of digits”. The mechanisms are complicated.) [4, 6, 17, 26, 29, 30]. The unopposed influence of GLI3R is accompanied by a marked increase in apoptosis and an increase in bone morphogenetic protein 4 (Bmp4), a known modulator of apoptosis [4]. The consequence of unopposed GLI3R is evident in the Shh knockout mouse [6] and in the spontaneous chicken deletion mutant (oligozeugodactyly) that is missing the ZPA regulatory region and thus has a loss of limb-specific SHH function [25]. In both of these mutants, the forelimbs have a humerus and radius but lack an ulna and digits (Fig. 2A). Conversely, loss of GLI3 function results in a decrease in apoptosis and marked polydactyly (with seven to 10 digits) [2, 40] (Fig. 2B). Thus, the current data from animal models suggest that one of the roles of GLI3R is to reduce the number of cells in the presumptive thumb region through targeted apoptosis mediated by BMP4.
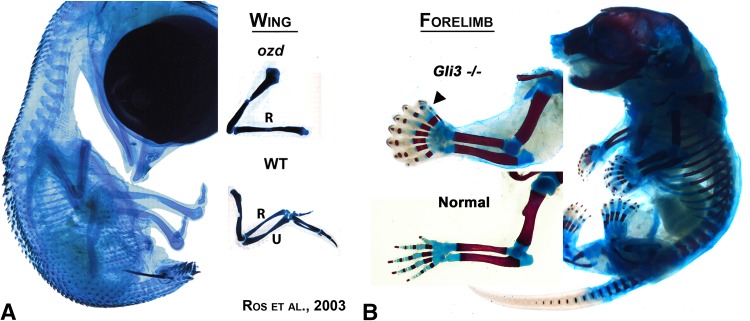
Fig. 2A–B.
The influence of GLI3 on digit formation. This figure illustrates the range of GLI3 influence in the two most common models used in limb development, chick and mouse. In the chicken deletion mutant Oligozeugodactyly (ozd) (A), the limb-specific enhancer of SHH has been deleted. Without SHH, GLI3R function is unopposed and inhibits the formation of the ulna and digits. In contrast, in the GLI3 knockout mouse (B) that lacks GLI3 function, excessive digits are formed (note eight digits) and these digits lack specific AP (radioulnar) identity, most conspicuously the absence of the two phalangeal thumb identity. (Images courtesy of John F. Fallon, PhD, University of Wisconsin, and Maria A. Ros, MD, PhD, Instituto de Biomedicina y Biotechnologia de Cantabria; (A) adapted with permission from Ros et al. [25].).
Establishing Digit Morphology
Cell death may highlight the region of the presumptive thumb, but other factors are needed to pattern thumb morphology. HOXA13, the most distal (also called 5′) gene of the HOXA cluster, is a well-established marker of the developing handplate and its initial expression tightly correlates with handplate formation [35, 37]. Mice lacking HOXA13 function fail to develop a digit 1 and exhibit repressed carpal and metacarpal formation [10, 28]. In contrast, overexpression of HOXA13 in the chick model transforms the bones of the zeugopod (forearm) into smaller bones that have carpal-like features and occasionally induces thumb duplication [36]. In humans, mutation in HOXA13 causes the hand-foot-uterus syndrome typically with hypoplastic and proximally displaced thumbs [11].
The T-box containing transcription factor 5 (TBX5) is expressed in the presumptive upper limb field and is responsible for upper limb bud initiation. TBX5 persists throughout the limb bud until handplate formation [1, 20, 24]. During handplate morphogenesis, TBX5 extends from the zuegopod (forearm) into the presumptive carpals and thumb but not into the phalanges of digits 2 to 5 (index through little finger) [15]. TBX5 is believed to play an important role in soft tissue patterning of the upper limb in general and within the thumb; in addition to soft tissue patterning, TBX5 appears to play an important role in limiting the thumb skeleton length [13, 15]. Mice missing one copy of TBX5 have longer thumbs, and in humans, disruption of TBX5 (Holt-Oram syndrome) is associated with a spectrum of musculoskeletal limb and thumb defects including triphalangeal thumbs [3, 15, 27]. Other factors such as SALL1/4 (which are associated with Townes-Brocks syndrome [OMIM #107480] and Duane-radial ray syndrome [OMIM #607323], respectively) are also present in the thumb and interact with TBX5 to modulate thumb length [15]; however, their expression and effect are not exclusive to the thumb domain.
SHH in the posterior or ulnar margin of the limb bud promotes limb expansion and patterning of the ulnar digits (index through little finger) [31, 39], whereas the development of the thumb or digit 1 is considered SHH-independent [12]. SHH directs patterning of the dependent digits by regulating the expression of distal HOXD transcription factors (HOXD10–13), which appear to expand or elongate the cartilage condensations that will form digits [35]. SHH manifests its action through the GLI3 transcription factor, inhibiting the truncation of GLI3 into its repressor form, thereby generating a GLI3 activator/repressor gradient [34, 38]. In addition to apoptosis, GLI3R has a role in refining digit morphology [5]. The distal HOXD transcription factors can interact directly with GLI3R to form an activator complex which presumably targets genes that direct digit elongation and specific morphogenesis [5]. Surprisingly, in the absence of GLI3, thumb morphology is lost and the handplate forms seven to 10 digits with similar indeterminate identity [17, 34] (Fig. 2B). In humans, GLI3 mutations can generate broadened or duplicated thumbs (Greig cephalopoly-syndactyly, OMIM #174700), but because of its roles in digit reduction and later refining digit morphologies, a number of additional phenotypes have also been reported (Pallister-Hall and polydactyly, preaxial, type IV, OMIM #175700, 146510).
The distal HOXD genes are initially expressed in a nested colinear pattern before handplate formation [38]. In other words, HOXD10 has the broadest initial expression domain within the limb bud. The expression of each successive HOXD gene (HOXD11–HOXD13) has a smaller, more posteriorly restricted expression domain nested within the previous genes’ domain. HOXD13 has the smallest domain, overlapping the ZPA. Once the handplate has formed, a second SHH-regulated phase of HOXD expression occurs that to some extent flips the expression domains, ie, reversed colinearity. HOXD13 has the most expansive expression domain extending to all of the presumptive digits, whereas HOXD10–12 are restricted to digits 2 to 5 [19] and exhibit similar domains of expression (see Fig. 1). The absence of HOXD10–12 expression within the anterior developing handplate is a marker of digit 1 identity across species [32, 33]. Initially the expression of HOXD13 is similar to HOXD10–12, being restricted to digits 2 to 5, but as the handplate develops, HOXD13 expression expands into the presumptive thumb domain [19]. Point mutations in human HOXD13 generate broadened thumbs (brachydactyly type D and E, OMIM #113300 and 113200). It is important to note that both HOXA13 and HOXD13 are expressed throughout the handplate and are essential for its development. In a double mouse knockout for both HOXA13 and HOXD13, no digits form and only a knob of cartilage is present at the end of the radius and ulna similar to a truncation defect [10].
Summary
The picture that emerges from the collective data is that the thumb is formed by a concerted and progressive interplay of transcription factors. The HOXA13 transcription factor specifies the handplate character and the induction of carpal-type condensations that will become the foundation of the thumb. GLI3 initially removes excess cells from the anterior handplate proximal to the presumptive thumb domain by apoptosis. Extension of the HOXD13 transcription factor into the thumb domain diverts GLI3 repression of digits by initiating a HOXD13-GLI3R collaboration to form and lengthen cartilage condensations into phalanges. This expansion is countered by TBX5, restraining the thumb length. Collectively these interactions yield the shortened thumb with only proximal and distal phalanges.
In some ways, the thumb, arguably one of the most defining human characteristics of the hand, appears to be a vestigial digit. It is the last digit to form. It lacks a middle phalanx. It is devoid of the digit empowering distal HOXD10–12 transcription factors. It is independent of the potent patterning influence of SHH. Indeed, at present, it is defined more by what molecules are lacking than what are present. Perhaps this somewhat independent environment also allows for greater adaptability. Across the animal kingdom, from moles to pandas, the thumb domain has given rise to a variety of morphologies and functions. To uncover the molecular basis of the uniquely mobile and opposable thumb of the human hand, animal models alone are insufficient; further research in clinical genetics will be required.
Acknowledgments
I thank Virchel Wood and the members of the Congenital Hand Anomalies Study Group (CHASG) for helpful discussions and providing clinical insights on the topic. I also thank Marian Ros for review, critical comments, and access to the GLI3 mutant mouse embryos.
Footnotes
The author certifies that he, or a member of his immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- 2.Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- 3.Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 4.Bastida MF, Delgado MD, Wang B, Fallon JF, Fernandez-Teran M, Ros MA. Levels of GLI3 repressor correlate with Bmp4 expression and apoptosis during limb development. Dev Dyn. 2004;231:148–160. doi: 10.1002/dvdy.20121. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Knezevic V, Ervin V, Hutson R, Ward Y, Mackem S. Direct interaction with Hoxd proteins reverses GLI3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–2347. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]
- 6.Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 7.Eldredge N, Gould SJ. Punctated equilibria: an alternative to phyletic gradualism. In: Schopf TJM, ed. Models in Paleobiology. San Francisco, CA, USA: Freeman Cooper & Co; 1972:82–115.
- 8.Fernandez-Teran MA, Hinchliffe JR, Ros MA. Birth and death of cells in limb development: a mapping study. Dev Dyn. 2006;235:2521–2537. doi: 10.1002/dvdy.20916. [DOI] [PubMed] [Google Scholar]
- 9.Frobisch NB, Carroll RL, Schoch RR. Limb ossification in the Paleozoic branchiosaurid Apateon (Temnospondyli) and the early evolution of preaxial dominance in tetrapod limb development. Evol Dev. 2007;9:69–75. doi: 10.1111/j.1525-142X.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 10.Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 11.Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, Holmes LB, Donnenfeld AE, Feingold M, Beemer FA, Hennekam RC, Scambler PJ. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. 2000;67:197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, Mohun TJ, Logan MP. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18:148–156. doi: 10.1016/j.devcel.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura S, Yamada S, Naruse I. Normal location of thumb/big toe may be related to programmed cell death in the preaxial area of embryonic limb. Anat Rec (Hoboken). 2011;294:1352–1359. doi: 10.1002/ar.21434. [DOI] [PubMed] [Google Scholar]
- 15.Koshiba-Takeuchi K, Takeuchi JK, Arruda EP, Kathiriya IS, Mo R, Hui CC, Srivastava D, Bruneau BG. Cooperative and antagonistic interactions between Sall4 and Tbx5 pattern the mouse limb and heart. Nat Genet. 2006;38:175–183. doi: 10.1038/ng1707. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Tickle C. Retinoic acid and pattern formation in the developing chick wing: SEM and quantitative studies of early effects on the apical ectodermal ridge and bud outgrowth. J Embryol Exp Morphol. 1985;90:139–169. [PubMed] [Google Scholar]
- 17.Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and GLI3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- 18.Milaire J. A new interpretation of the necrotic changes occurring in the developing limb bud paddle of mouse embryos based upon recent observations in four different phenotypes. Int J Dev Biol. 1992;36:169–178. [PubMed] [Google Scholar]
- 19.Montavon T, Le Garrec JF, Kerszberg M, Duboule D. Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev. 2008;22:346–359. doi: 10.1101/gad.1631708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng JK, Kawakami Y, Büscher D, Raya A, Itoh T, Koth CM, Rodríguez Esteban C, Rodríguez-León J, Garrity DM, Fishman MC, Izpisúa Belmonte JC. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129:5161–5170. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- 21.O’Rahilly R, Muller F. Developmental Stages in Human Embryos. Washington, DC, USA: Carnegie Institution of Washington; 1987. [Google Scholar]
- 22.Raynaud A. Developmental mechanism involved in the embryonic reduction of limbs in reptiles. Int J Dev Biol. 1990;34:233–243. [PubMed] [Google Scholar]
- 23.Raynaud A, Clergue-Gazeau M. [Chemically induced ectropodia in the Lacerta viridis embryo and formation of styliform limbs in reptiles] [in French] C R Acad Sci III. 1984;298:457–460. [PubMed] [Google Scholar]
- 24.Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398:814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- 25.Ros MA, Dahn RD, Fernandez-Teran M, Rashka K, Caruccio NC, Hasso SM, Bitgood JJ, Lancman JJ, Fallon JF. The chick oligozeugodactyly (ozd) mutant lacks sonic hedgehog function in the limb. Development. 2003;130:527–537. doi: 10.1242/dev.00245. [DOI] [PubMed] [Google Scholar]
- 26.Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spranger S, Ulmer H, Tröger J, Jansen O, Graf J, Meinck HM, Spranger M. Muscular involvement in the Holt-Oram syndrome. J Med Genet. 1997;34:978–981. doi: 10.1136/jmg.34.12.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128:4177–4188. doi: 10.1242/dev.128.21.4177. [DOI] [PubMed] [Google Scholar]
- 29.Stopper GF, Wagner GP. Inhibition of Sonic hedgehog signaling leads to posterior digit loss in Ambystoma mexicanum: parallels to natural digit reduction in urodeles. Dev Dyn. 2007;236:321–331. doi: 10.1002/dvdy.21025. [DOI] [PubMed] [Google Scholar]
- 30.te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, Zeller R. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- 31.Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- 32.Vargas AO, Fallon JF. The digits of the wing of birds are 1, 2, and 3. A review. J Exp Zoolog B Mol Dev Evol. 2005;304:206–219. doi: 10.1002/jez.b.21051. [DOI] [PubMed] [Google Scholar]
- 33.Vargas AO, Kohlsdorf T, Fallon JF, Vandenbrooks J, Wagner GP. The evolution of HoxD-11 expression in the bird wing: insights from Alligator mississippiensis. PLoS One. 2008;3:e3325. doi: 10.1371/journal.pone.0003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of GLI3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/S0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 35.Woltering JM, Duboule D. The origin of digits: expression patterns versus regulatory mechanisms. Dev Cell. 2010;18:526–532. doi: 10.1016/j.devcel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Yokouchi Y, Nakazato S, Yamamoto M, Goto Y, Kameda T, Iba H, Kuroiwa A. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995;9:2509–2522. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- 37.Yokouchi Y, Sasaki H, Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991;353:443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- 38.Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuniga A, Zeller R. GLI3 (Xt) and formin (ld) participate in the positioning of the polarising region and control of posterior limb-bud identity. Development. 1999;126:13–21. doi: 10.1242/dev.126.1.13. [DOI] [PubMed] [Google Scholar]