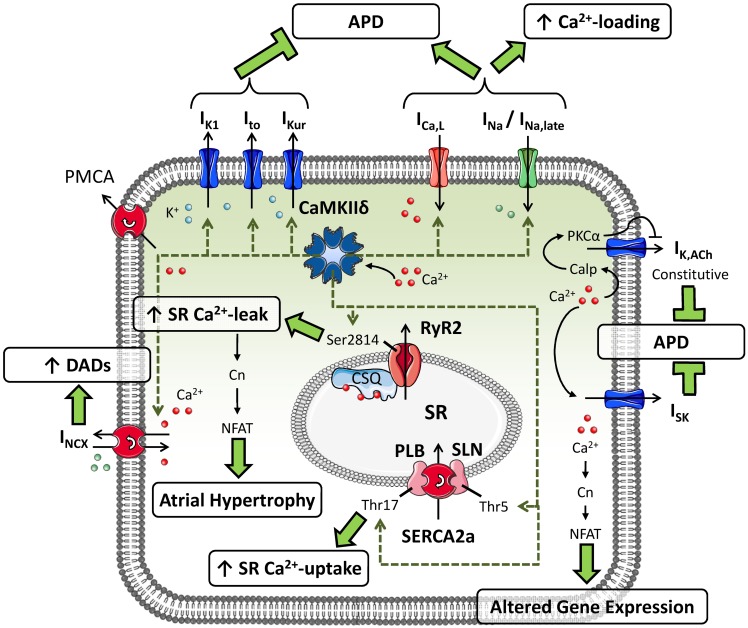
Figure 1.
Putative substrates for CaMKII-dependent phosphorylation in atrial cardiomyocytes and their consequences for atrial cellular electrophysiology and Ca2+-handling. CaMKII can phosphorylate the transient-outward K+-current (Ito), inward-rectifier K+-current (IK1) and ultra-rapid delayed-rectifier K+-current (IKur), augmenting their functions and shortening action potential duration (APD). Phosphorylation of L-type Ca2+-current (ICa,L) and Na+-current (INa; resulting in an increased late component: INa,late) by CaMKII increases intracellular Ca2+ levels and prolongs APD. CaMKII-dependent phosphorylation of phospholamban (PLB) and sarcolipin (SLN) increases sarcoplasmic reticulum (SR) Ca2+-uptake, whereas phosphorylation of type-2 ryanodine-receptor channels (RyR2) promotes diastolic SR Ca2+-leak. CaMKII-dependent increases in expression of Na+/Ca2+-exchanger type-1 (NCX1) augment NCX-current (INCX), promoting the occurrence of delayed afterdepolarizations (DADs). In addition, Ca2+-handling abnormalities can activate small-conductance Ca2+-activated K+-currents (ISK) and agonist-independent “constitutive” IK,ACh, shortening APD, and promote altered gene expression via the Ca2+-dependent phosphatase calcineurin (Cn).