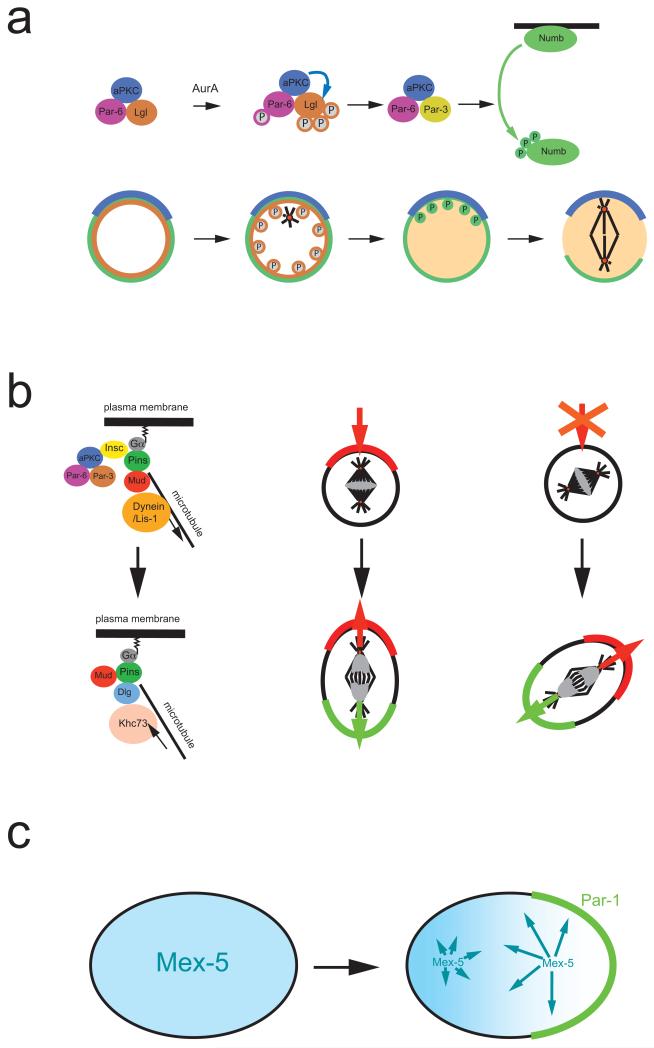
Figure 2. Asymmetric segregation of protein determinants.
a ∣ In Drosophila melanogaster neuroblasts, activation of the kinase Aurora A triggers a subunit exchange in the apically localized Par-3–aPKC complex. Aurora A phosphorylates Par-6, which in turn activates aPKC, leading to Lgl phosphorylation and exit from the complex. Lgl is exchanged for Par-3, which acts as an adaptor that allows aPKC to phosphorylate of Numb. Phosphorylated Numb is released into the cytoplasm. Since aPKC is restricted to the apical cortex, Numb is retained on the basal side and segregates into the basal daughter cell.
b ∣ Left side: In metaphase, Gα, Pins and Mud establish a cortical attachment site for astral microtubules to orient the mitotic spindle. In telophase, however, it is the mitotic spindle that influences cortical polarity via a pathway involving the kinesin Khc-73 and the protein Dlg. Right side: Normally, the telophase pathway is not essential. When components of the apical complex are missing, however, it will rescue the formation of opposing cortical domains in ana and telophase. The new polarity axis aligns with the mitotic spindle and no longer always with apical-basal polarity.
c ∣ In C. elegans, Mex-5 (blue) and Pie-1 (not shown) exist as fast and slow diffusing forms. The faster diffusing form of Mex-5 is more abundant posteriorly while more rapidly diffusable Pie-1 is anterior, resulting in the asymmetric distribution of the cytoplasmic proteins. For Mex-5, phosphorylation by posteriorly localized Par-1 may be responsible for the faster diffusion rate.