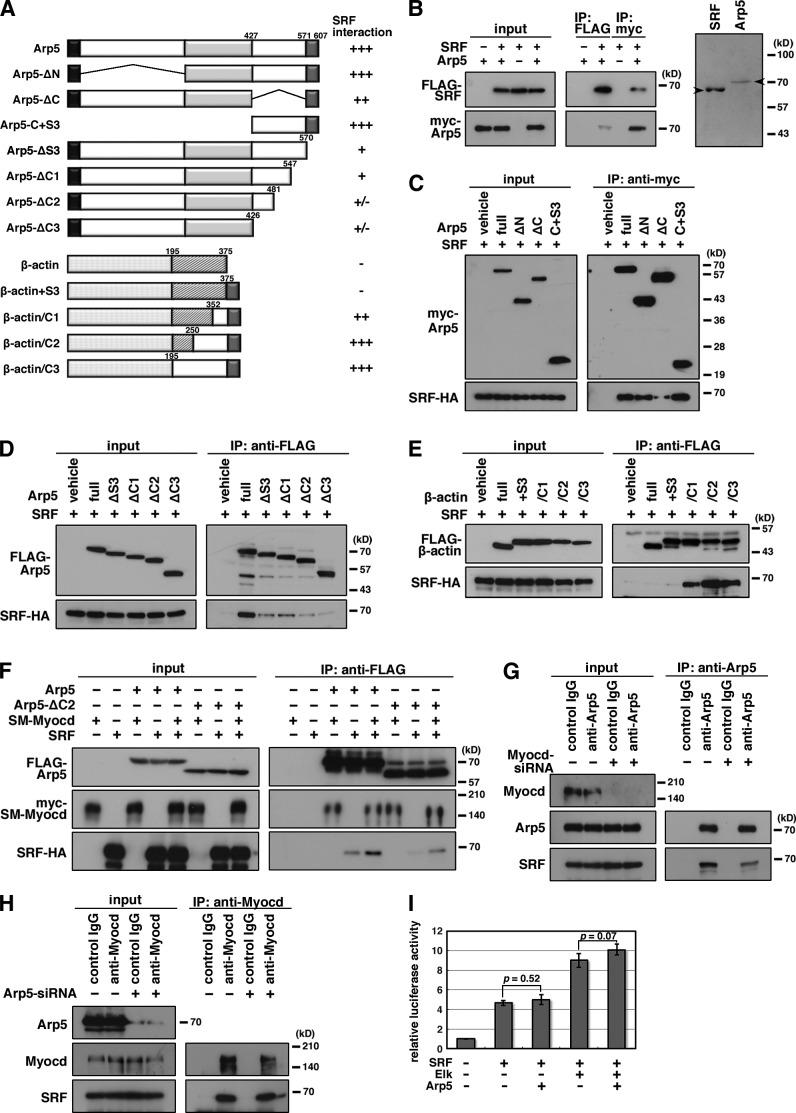
Figure 5.
Mapping the interaction domain of Arp5 with SRF. (A) Schematic representation of the deletion and chimeric constructs of Arp5 and β-actin. Numbers indicate amino acid positions. (B) The direct interaction between SRF and Arp5 was determined by coimmunoprecipitation using bacterially synthesized recombinant SRF and Arp5 proteins (left). Purified proteins were visualized on a Coomassie-stained gel (right). Arrowheads indicate the positions of full-length recombinant SRF and Arp5 proteins. (C–F) Hek293T cells were transfected with the indicated combinations of expression vectors and coimmunoprecipitation was performed. (G and H) A7r5 cells were transfected with Myocd siRNA (G) or Arp5 siRNA1 (H) for 3 d, after which a coimmunoprecipitation assay was done using the indicated antibodies. (I) The indicated expression vectors were transfected into HeLa cells with the c-fos promoter reporter construct, and the luciferase reporter assay was performed. Data represent the mean ± SEM of four independent experiments. P-values were calculated using Student’s t test.