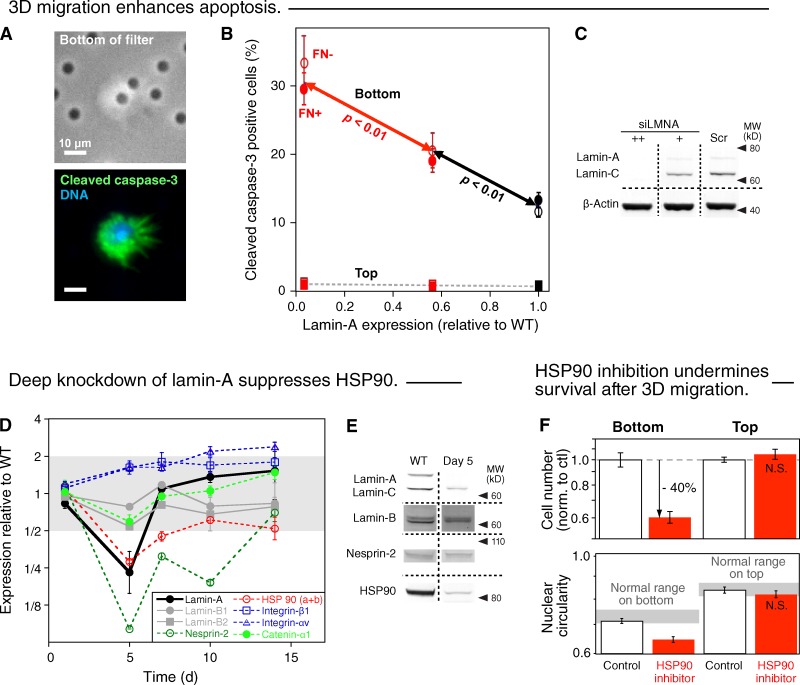
Figure 4.
3D migration enhances apoptosis, and ablation of lamin-A compromises HSP90-dependent stress protection. (A) Images of A549 cell partially in a 3-µm pore (top) and staining positive for the apoptosis marker cleaved caspase-3 (green, bottom; blue, DNA Hoechst 33342). (B) Increased frequency of apoptotic A549 cells on the bottom of 3-µm filter after lamin-A knockdown. Cells on top show a negligible dependence of apoptosis on lamin-A. (n = 3; ±SEM). (C) Measurements were made by immunoblotting. (D) Changes in the expression levels of proteins of interest, detected by quantitative mass spectrometry, after transient lamin-A knockdown and recovery over 14 d (n = 3; ±SEM). Although up-regulation of the fibronectin receptor integrin α5:β1 in epithelial cells can favor mesenchymal-like motility (Friedl et al., 1997), correlations of levels with extents of cell behaviors remain controversial (Desgrosellier and Cheresh, 2010) and, more importantly, FN coating of filters had no effect in our studies (Fig. 1, D and E). Likewise, while decreases in α-catenin (by > 25%) also associate with an epithelial-mesenchymal transition (EMT; Yang et al., 2004), deep lamin-A knockdown suppresses net 3D migration (Fig. 1 D). (E) Changes in lamin-A, lamin-B, nesprin-2, and HSP90 were confirmed by immunoblot for wild-type and 5-d post-knockdown cells. (F) A549 cells pretreated for 24 h with HSP90 inhibitor (17-AAG, 90 nM in DMSO) or the same volume of DMSO (control) were plated in the same solutions on the top of 3-µm filters and allowed to migrate for 24 h. Cells were fixed, immunostained for lamin-A, imaged, and measured (n = 3; ±SEM); drug gave fewer cells on bottom and nuclei were less circular (P < 0.01; log scales).