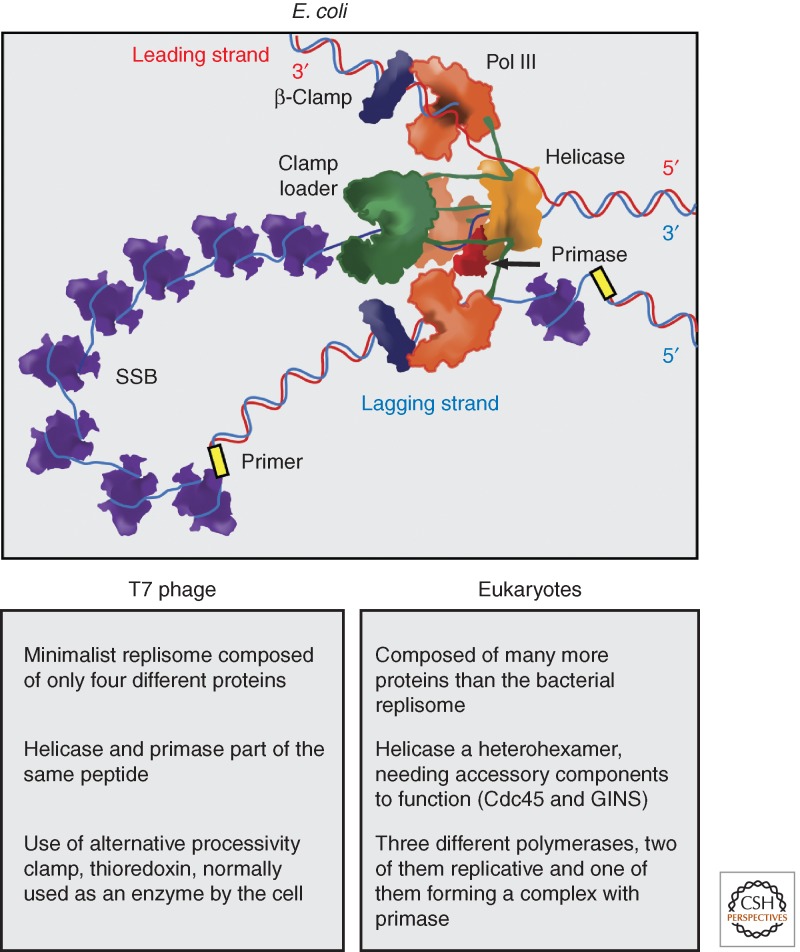
Figure 4.
Replisome architecture. The arrangement of the different components of the E. coli replisome is shown. At the front of the replication fork, a hexameric helicase melts the dsDNA. Helicase binds primase, and it is connected to the three replicative polymerases (Pol III) via the τ subunit of the clamp loader. A leading-strand polymerase travels in the same direction of the fork, while the two remaining polymerases can potentially act on the lagging strand. Each active polymerase is bound to the homodimeric β-clamp, which acts as a processivity factor. The leading strand is synthesized continuously, whereas the lagging strand requires cycles of chain elongation. Progression of helicase causes the accumulation of ssDNA, which is covered by the homotetrameric SSB. The clamp loader (heteropentamer) loads β-clamp, mediates primase handover to polymerase, and connects the polymerase with helicase. The architecture of the replisome in other systems is thought to be similar to that of E. coli (Yao and O’Donnell 2010). Some differences with phage and eukaryote replisomes are listed.