Fig. (4).
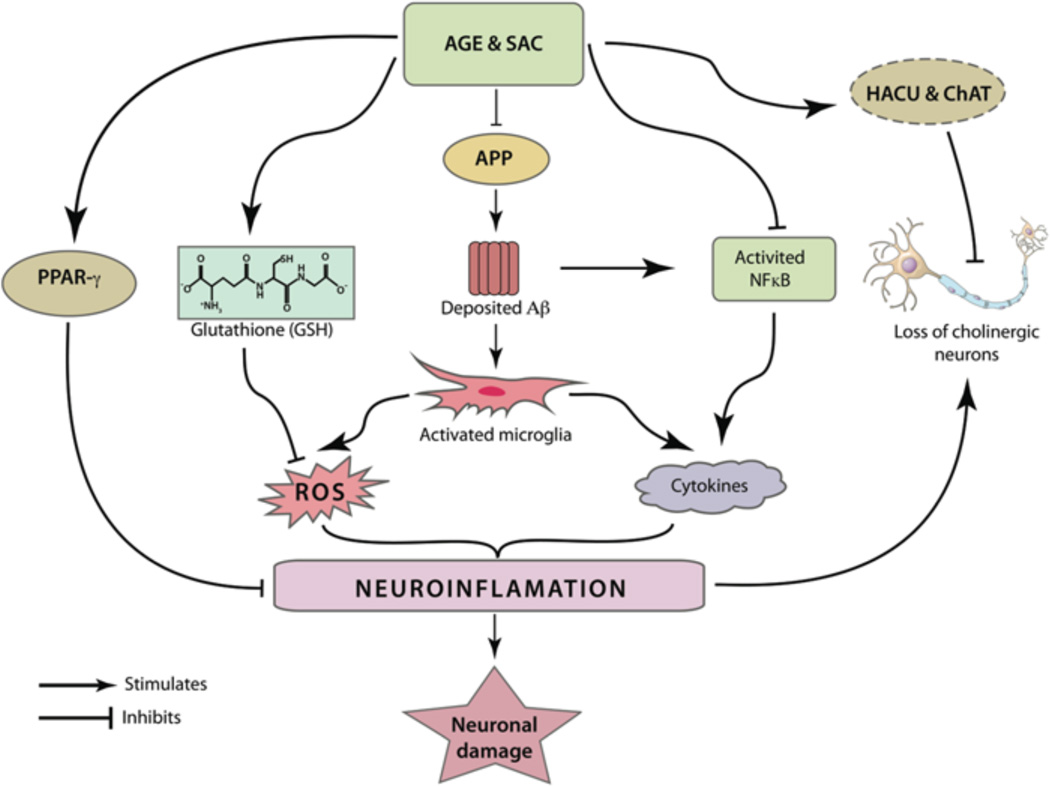
AGE and SAC can modulate different inflammatory pathways related to the pathogenesis of AD. Microglial activation by deposited Aβ peptides can be abridged by their potential roles in reducing the brain Aβ load. Further, SAC was found to inhibit the activation of the pro-inflammatory transcription factor NFκB. The latter plays an important role in the production of several cytochemokines. Activated NFκB is also responsible in generation of Aβ from APP. As mentioned in the text, apart from producing cytochemokines, activated microglia can produce ROS, leading to widespread neuronal damage. AGE and SAC treatments were independently shown to increase the production of glutathione (GSH), an important molecule to neutralize ROS. AGE treatment was also shown to have PPAR-γ agonistic property and the latter is believed to modulate expression of several pro-inflammatory genes. It was postulated that cholinergic neurons are most vulnerable by inflammation associated with AD. Notably, the increased HACU and ChAT activity, as observed by AGE and SAC treatment, is able to preserve cholinergic neurons from ROS or cytochemokines-mediated damage.