Fig. 6.
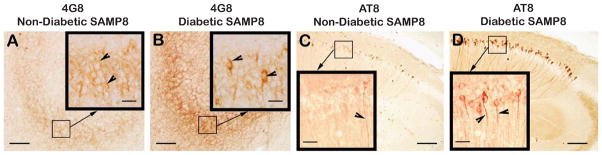
Immunodistribution of 4G8 (A, B) and phospho-tau (AT8) (C, D) in the hippocampus of low-fat diet fed non-diabetic SAMP8 mice (A, C), and in the hippocampus of high-fat diet fed diabetic SAMP8 mice (B, D). Note faint 4G8 immunoreactivity within the perikarya of CA3 granule cells (Fig. 6A, inlet, arrowheads) of non-diabetic SAMP8 mice, indicating the presence of intraneuronal Aβ accumulated as a result of accelerated aging in absence of diabetes. Note stronger 4G8 immunoreaction within the perikarya of CA3 granule cells (Fig. 6B, inlet, arrowheads) of diabetic SAMP8 mice, indicating increased accumulation of intraneuronal Aβ than that of non-diabetic SAMP8 mice increased as a result of accelerated aging in presence of diabetes. Similarly, there was observed some evidence of tau phosphorylation within the CA1 hippocampal neurites in non-diabetic SAMP8 mice (Fig. 6C, inlet, arrowhead) merely due to accelerated aging, which was remarkably increased in the CA hippocampal CA1 of diabetic SAMP8 mice (Fig. 6D, inlet, arrowheads) as a result of accelerated aging compounded with diabetes. Scale bars in A-D = 100 μm; Scale bars in all inlets = 20 μm.
