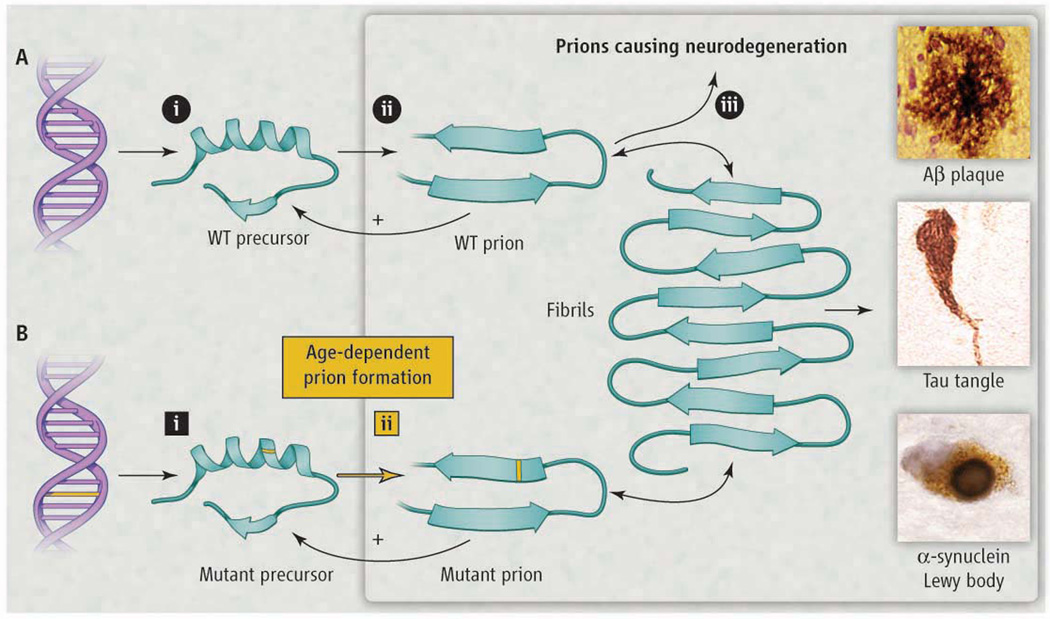
Figure. Modeling Neurodegeneration Caused by Prions.
(A) Prions multiply through self-propagating cycles of post-translational modification; generally, an increase in β-sheet content accompanies prion formation. Pathogenic prions are most toxic as oligomers and less toxic after polymerization into amyloid fibrils. Depending on the protein, the fibrils coalesce into amyloid plaques, neurofibrillary tangles, or intracellular inclusions such as Lewy or Pick bodies. Drug targets for the development of therapeutics (octagons): 1) lowering precursor protein, 2) inhibiting prion formation, and 3) enhancing prion clearance. (B) Lateonset heritable neurodegeneration argues for two discrete events (squares): 1) mutation and 2) prion formation.