Abstract
BACKGROUND
Pulmonary non-tuberculous mycobacterial (NTM) infection is relatively common after lung transplantation, but the effect on mortality remains undetermined. Herein we describe our experience with pulmonary NTM infection after lung transplantation and hypothesized that non-tuberculous mycobacterial infection after lung transplantation would be associated with increased mortality.
METHODS
We retrospectively evaluated 201 primary lung transplant recipients transplanted between January 2000 and August 2006. Serial bronchoscopies with bronchoalveolar lavage and transbronchial biopsy were performed according to a surveillance protocol and when clinically indicated. The diagnosis NTM infection was established by a positive NTM culture in a bronchoalveolar lavage sample or in at least two separate expectorated sputum samples. NTM infections were further classified as “disease” or “colonization,” based on whether or not NTM infection patients developed symptoms and characteristic radiographic findings.
RESULTS
Thirty-six (18%) recipients were diagnosed with pulmonary NTM infection at a median of 97 days post-transplantation: 9 were classified as NTM disease and the remaining 27 as NTM colonization cases. Single lung transplant was a significant risk factor for NTM infection (HR 2.25, p = 0.02). NTM colonization was a risk factor for NTM disease (HR 8.39, p = 0.003). NTM infection significantly increased the risk of death after lung transplantation (HR 2.61, p = 0.001) and persisted in multivariate models controlling for single lung transplant and bronchiolitis obliterans syndrome. The increased risk was seen for both NTM colonization and NTM disease. Among the patients who died, non-NTM infection was a more common contributing factor in the cause of death for the NTM infection group (44% vs 12%, p = 0.04).
CONCLUSIONS
Non-tuberculous mycobacterial infection is common after lung transplantation. NTM colonization and treated acute rejection are risk factors for NTM disease. NTM infection is associated with increased risk of mortality independent of bronchiolitis obliterans syndrome.
Keywords: non-tuberculous mycobacterium, lung transplantation, infection, mortality, outcomes
The median survival after lung transplantation is 5.3 years, as reported by the registry of the International Society for Heart and Lung Transplantation (ISHLT) in 2010, which is significantly shorter than the survival duration reported for other types of solid-organ transplant recipients.1 Although infection is a major direct cause of early and late morbidity and mortality after lung transplantation, many infections also have been implicated as risk factors for the development of bronchiolitis obliterans syndrome (BOS) and, consequently, indirectly limit long-term survival after lung transplantation.2–17
The spectrum of pulmonary non-tuberculous mycobacterial (NTM) infection after lung transplantation has been described previously18–28; however, the effect of NTM on allograft function and mortality remains undetermined. In this study, we describe our experience with NTM infections in a large, single-center lung transplant cohort, and in our retrospective analysis we found that NTM infection is a risk factor for mortality in lung transplant recipients.
Methods
Recipient cohort
This study cohort consisted of all primary adult lung transplant recipients transplanted at UCLA between January 1, 2000 and August 30, 2006. Of the 207 consecutive lung transplant operations performed, 201 recipients met the inclusion criteria and their follow-up data were collected through December 2009. Five retransplant recipients were excluded from the final cohort. One additional patient with intra-operative death was also excluded. The study was approved by the institutional review board at UCLA.
Standard care of lung transplant recipients
Post-transplant immunosuppression and anti-microbial prophylaxis was administered according to protocols of the UCLA lung transplant program, as previously described.15 Briefly, patients >65 years of age, or those <65 years of age with history of malignancy or an increased risk of infection as perceived by the transplant physician, received induction therapy with basiliximab 20 mg intravenously (IV) on Days 1 and 4 post-operatively. All other patients received three doses of rabbit anti-thymocyte globulin (ATG) 1.5 mg/kg IV on Days 0, 1 and 2 post-operatively. Patients were generally maintained on triple-drug immunosuppression with corticosteroids, tacrolimus and mycophenolate mofetil (MMF).
All recipients with positive serology for cytomegalovirus (CMV) and those with serologically positive donors received IV ganciclovir (3 mg/kg/day) for 2 weeks, followed by oral valganciclovir (900 mg/day) for at least 6 months. Trimethoprim–sulfamethoxazole (double-strength tablet every 12 hours, 2 days per week) was given indefinitely for Pneumocystis jiroveci prophylaxis. Fungal prophylaxis included nebulized amphotericin B 15 mg twice daily (January 2000 to September 2003) or nebulized amphotericin B lipid complex 50 mg/day (October 2003 to present) for 3 days and then weekly for the duration of the immediate post-transplant hospitalization for all patients. Recipients with a history of pre-transplant fungal infection were treated with an additional 6-month course of either itraconazole 200 mg twice daily (January 2000 to May 2003) or voriconazole 200 mg twice daily (June 2003 to present), which was started prior to discharge from the hospital.
Patients underwent surveillance bronchoscopy within the first 24 hours, at Week 1, and at Months 1, 3, 6 and 12 post-transplantation. Clinically indicated bronchoscopy was also performed for new respiratory symptoms, radiographic findings, >10% decline in forced expiratory volume in 1 second (FEV1), or to monitor for treatment responses after a prior abnormal bronchoscopy.
Definition of NTM infection, colonization and disease and treatment
The diagnosis of NTM infection in this study required one positive culture from a BAL sample or at least two positive sputum cultures for NTM. Patients diagnosed with NTM infection were further sub-grouped based on whether or not they ever developed NTM disease. In accordance with the 2007 ATS/IDSA statement, we defined NTM disease as the presence of pulmonary symptoms, nodular or cavitary opacities on chest radiograph or computed tomography (CT) and meeting criteria for NTM infection.29 Those patients with NTM infection who never developed NTM disease were classified as NTM-colonization cases. Quantitative cultures are not performed at our center and were therefore unavailable. We considered the isolation of Mycobacterium gordonae as contamination in accordance with the 2007 ATS/IDSA statement.29
Once diagnosed, NTM disease was always treated for a duration of at least 6 months after the conversion to a negative culture. In general, NTM colonization was untreated and these recipients were closely observed for signs of the development of NTM disease. However, indications for treating NTM infection included recurrent isolation of the same NTM species and infection with Mycobacterium abscessus. Initial treatment generally consisted of three antibiotic medications directed at the specific NTM species and were adjusted based on available sensitivities and the clinical course.
Definition of acute rejection and BOS
Acute rejection (AR) was diagnosed and graded by a pathologist experienced in lung transplantation and in accordance with standard ISHLT criteria.30 The cumulative AR score was derived by summing all “A” grades occurring in an individual prior to a specified time-point or event (i.e., 180 days, BOS, etc.). BOS (defined as Stage 1 or higher) was diagnosed by a sustained drop in FEV1 from the post-transplant baseline as defined by ISHLT criteria.31 A diagnosis of BOS was made only after exclusion of other causes of allograft dysfunction.
Statistical analysis
Descriptive statistics for recipient and donor characteristics included mean ± standard deviation (SD) or median with the interquartile range (IQR), as appropriate. Categorical variables are presented as proportions. Continuous variables were analyzed using non-parametric t-tests and categorical data were analyzed by chi-square or Fisher’s exact tests. Count data (i.e., number of bronchoscopies performed) were analyzed with Poisson regression.
Univariate Cox regression models were used to analyze for risk factors for NTM infection and NTM disease and to assess the relationship between NTM and mortality or BOS. For mortality and BOS, final multivariate models were adjusted for single lung transplantation based on differences between NTM and non-NTM groups. A priori and based on existing knowledge, we chose to include BOS in the final models for mortality and the cumulative AR score in the final models for BOS. Post-transplant events were analyzed as time-dependent variables to avoid assignment of risk before their occurrence.
Time-dependent variables were constructed as an interaction between time and variable status. For example, the NTM infection variable takes the value of 1 or 0 at time t (measured from the date of transplantation), depending on whether or not the patient developed NTM infection by that time. The proportional hazards assumption was satisfied by all time-independent variables as determined by various residual analyses.
Results
Study cohort characteristics
The final study cohort included 201 primary lung transplant recipients (65 single, 133 bilateral, 3 heart–lung). The NTM infection group (n = 36) included all patients diagnosed with NTM colonization (n = 27) or NTM disease (n = 9) at any point after lung transplantation. The non-NTM group (n = 165) consisted of all the remaining patients, including patients with a BAL culture positive for M gordonae (n = 4), which was considered to be a contaminant in accordance with ATS/IDSA guidelines.29 Notably, no patient with a positive culture for M gordonae ever had evidence of NTM disease or persistent infection (i.e., recurrent isolation of M gordonae), and no patient ever received treatment directed at M gordonae.
Demographics and clinical characteristics of the study groups are summarized in Table 1. At the time of transplantation, only the type of lung transplant operation was significantly different between groups. During the follow-up period, the NTM disease group had more total bronchoscopies performed on average than both the non-NTM group and the NTM infection group. The non-NTM group and the NTM colonization group had similar numbers of bronchoscopies performed.
Table 1.
Characteristics of the Study Groups
| No NTM (n = 165) |
NTM colonization (n = 27) |
NTM disease (n = 9) |
p-value | |
|---|---|---|---|---|
| Male, n (%) | 87 (53) | 17 (63) | 5 (56) | 0.61 |
| Recipient age, years (mean ± SD) | 55.6 ± 10.3 | 59.7 ± 8.7 | 54.3 ± 9.8 | 0.13 |
| Donor age, years (mean ± SD) | 34.4 ± 15.1 | 40.7 ± 17.5 | 33.7 ± 13.6 | 0.14 |
| Pre-transplant disease, n (%) | – | |||
| Obstructive | 68 (41) | 14 (52) | 3 (33) | |
| Restrictive | 76 (46) | 13 (48) | 4 (44) | |
| CF/bronchiectasis | 14 (9) | 0 (0) | 0 (0) | |
| Vascular | 7 (4) | 0 (0) | 2 (22) | |
| Single lung transplant, n (%) | 48 (29) | 15 (56) | 2 (22) | 0.02b |
| Induction agent, n (%)a | 0.90 | |||
| ATG | 103 (62) | 16 (59) | 6 (67) | |
| Basiliximab | 60 (36) | 11 (41) | 3 (33) | |
| No. of bronchoscopies (median, IQR) | 5 (4–7) | 7 (4–8) | 11 (8–12) | <0.001c |
| Tx hospitalization, days (median, IQR) | 13 (10–20) | 11 (9–15) | 20 (10–24) | 0.14 |
| Follow-up, days (median, IQR) | 1,361 (525–1,920) | 1,225 (426–1,619) | 1,185 (532–1,643) | 0.71 |
ATG, anti-thymocyte globulin; CF, cystic fibrosis; IQR, interquartile range; NTM, non-tuberculous mycobacterium; TB, transbronchial; Tx, transplant.
Two patients from the No NTM group did not receive any induction therapy.
No NTM vs NTM colonization: p < 0.05.
No NTM vs NTM disease: p < 0.001; NTM colonization vs NTM disease: p < 0.05.
Pre-transplant and donor NTM
Recipient and donor pre-transplant cultures for NTM were not performed universally. Only 105 recipients (52%) had a pre-transplant NTM culture performed. Of those screened, 6 lung transplant recipients were positive for pre-transplant NTM. Four were diagnosed with M fortuitum infection prior to transplant and each received at least 3 months of appropriate therapy and remained on therapy up to the time of lung transplantation. None of these 4 recipients developed post-transplant NTM. The pre-transplant diagnosis was idiopathic pulmonary fibrosis (IPF) in 3 of these patients, and sarcoidosis in 1 patient. A fifth recipient had pre-transplant Mycobacterium avium complex disease and received >6 months of appropriate antibiotic therapy up to the time of single lung transplantation for IPF. This recipient received a single lung transplant and had repeated positive cultures for M avium complex post-transplant, with the initial positive isolate at 92 days post-transplant. A sixth recipient with chronic obstructive pulmonary disease (COPD) had a remote positive sputum culture for M avium complex, but bronchoalveolar lavage (BAL) done during the transplant evaluation was negative, and this patient never received therapy and never had a positive NTM culture post-transplant. Of those recipients who screened negative for pretransplant NTM infection (n = 99), 14 (14%) developed post-transplant NTM infection, 2 (2%) of whom developed post-transplant NTM disease.
Back-table swabs of donor lungs were screened for acid-fast bacilli (AFB) in only 69 cases (34%). Only 1 donor bronchial swab was positive for NTM (M fortuitum), and the recipient of this donor was placed on antibiotic therapy immediately post-transplant and never had a positive NTM culture post-transplant. Of those donor lungs that screened negative for NTM infection (n = 68), 14 (21%) developed post-transplant NTM infection, and 5 (7%) developed post-transplant NTM disease.
Spectrum of NTM infection and treatment after lung transplantation
Among the NTM-colonized group (n = 27), M avium complex accounted for the majority of post-transplant NTM isolates (Table 2). NTM colonization was diagnosed from a surveillance bronchoscopy in 19 patients (i.e., no new respiratory symptoms or radiographic changes). The other 8 cases of NTM colonization were diagnosed from a bronchoscopy prompted by new respiratory symptoms, but none of these cases had radiographic findings characteristic for NTM disease. The diagnoses in these cases were bacterial pneumonia (n = 3), bacterial bronchitis (n = 2), CMV pneumonitis (n = 1), severe primary graft dysfunction (PGD; n = 1) and atelectasis (n = 1). Only 4 cases of NTM colonization were treated: 2 with M avium complex; 1 with M abscessus; and 1 with M fortuitum. Subsequent respiratory cultures documented NTM clearance in 3 of these cases. In the fourth case (M avium complex), NTM clearance was not established because the patient died 1 month later of pneumonia caused by Pseudomonas aeruginosa. In the 23 untreated NTM colonization cases, all subsequent respiratory cultures were negative for NTM in 15, not performed in 6, and remained positive for NTM in only 2 patients.
Table 2.
NTM Species by Study Group
| NTM colonization (n = 27) |
NTM disease (n = 9) |
|
|---|---|---|
| M avium complex | 18 (67%) | 4 (44.5%) |
| M simiae | 4 (15%) | 1 (11%) |
| M abscessus | 1 (3.7%) | 4 (44.5%) |
| M chelonae | 2 (7%) | 0 (0%) |
| M fortuitum | 1 (3.7%) | 0 (0%) |
| M peregrinum | 1 (3.7%) | 0 (0%) |
NTM, non-tuberculous mycobacterium.
Among the NTM disease group (n = 9), M abscessus, along with M avium complex, was a dominant cause of NTM disease (Table 2). NTM disease was diagnosed from a symptom-indicated bronchoscopy in 7 cases and from sputum in 1 case. One additional NTM disease case was diagnosed with Day 1 post-operative BAL positive for M avium complex. In this right single lung transplant recipient, the explant pathology revealed a caseating granuloma that was culture-positive for M avium complex and the remaining left native lung had a left upper lobe (LUL) nodular opacity on CT. No patient developed disseminated disease. Table 3 summarizes the spectrum of NTM disease and response to treatment in these patients.
Table 3.
Spectrum of NTM Disease and Treatment
| Pt | Age (y) |
Gender | Diagnosis | Transplant type |
Days to infection diagnosis |
Days to disease diagnosis |
NTM species |
|---|---|---|---|---|---|---|---|
| 1 | 49 | F | IPF | BL | 614 | 614 | Abscessus |
| 2 | 53 | F | IPAH | BL | 323 | 323 | MAC |
| 3 | 55 | M | COPD | SL | 252 | 788 | MAC |
| 4 | 56 | F | SCL | BL | 8 | 487 | Abscessus |
| 5 | 70 | M | COPD | SL | 1 | 1 | MAC |
| 6 | 33 | M | SCL | BL | 583 | 583 | Abscessus |
| 7 | 56 | F | COPD | BL | 605 | 720 | MAC |
| 8 | 60 | M | IPAH | BL | 749 | 749 | Simaie |
| 9 | 57 | M | IPF | BL | 8 | 83 | Abscessus |
| Respiratory symptoms |
CT/CXR finding | Treatment | Duration (months) |
Response to treatment | Status/days post-operative |
|---|---|---|---|---|---|
| SOB, cough | Bilateral centrilobular nodularity | A, Ami, Imi→A | 12 | NTM clearance by 3 mo, progressive graft dysfunction | Dead/1,161 |
| Cough | Right centrilobular nodularity | C, ethambutol, rifabutin | 5 | NTM clearance by 3 mo, progressive graft dysfunction | Dead/452 |
| SOB, cough | RUL nodular opacity (native lung) | C, ethambutol, rifabutin | 18 | NTM clearance by 6 mo, complete resolution | Dead/1,606 |
| SOB | RLL centrilobular nodularity and consolidation | A, Ami, Imi→A | 24 | NTM clearance by 6 mo, complete resolution | Alive/2,109 |
| Post-operative | LUL nodular opacity (native lung) | A, ciprofloxacin | 12 | NTM clearance by 1 mo, complete resolution | Dead/1,185 |
| SOB, cough | RLL centrilobular nodularity and consolidation | C, Ami, tygecycline | 1 | Unknown NTM clearance, progressive graft dysfunction | Dead/612 |
| SOB, cough | Right centrilobular nodularity | A, ethambutol, ciprofloxacin→A | 12 | NTM clearance by 3 mo, progressive graft dysfunction | Alive/ 1,679 |
| SOB, pleurisy | LUL nodular opacity | A, moxifloxacin, rifabutin | 12 | NTM clearance by 3 mo, complete resolution | Alive/1,295 |
| Hypoxemia, cough | Clustered tree-in-bud centrilobular air-space nodularity | C, Ami | 9 | NTM clearance by 3 mo, progressive graft dysfunction | Dead/346 |
A, azithromycin; Ami, amikacin; BL, bilateral lung; C, clarithromycin; COPD, chronic obstructive pulmonary disease; F, female; Imi, imipenem; IPAH, idiopathic pulmonary arterial hypertension; IPF, idiopathic pulmonary fibrosis; LUL, left upper lobe; M, male; MAC, Mycobacterium avium complex; NTM, non-tuberculous mycobacterium; SCL, scleroderma; SL, single lung; SOB, shortness of breath; →, transitioned to.
In general, anti-microbial susceptibility testing was performed only when treatment was being considered, including for all NTM disease cases. When tested, NTM infections (n = 19), including M avium complex infections (n = 9), were universally susceptible to clarithromycin.
Time to and risk factors for NTM infection and disease
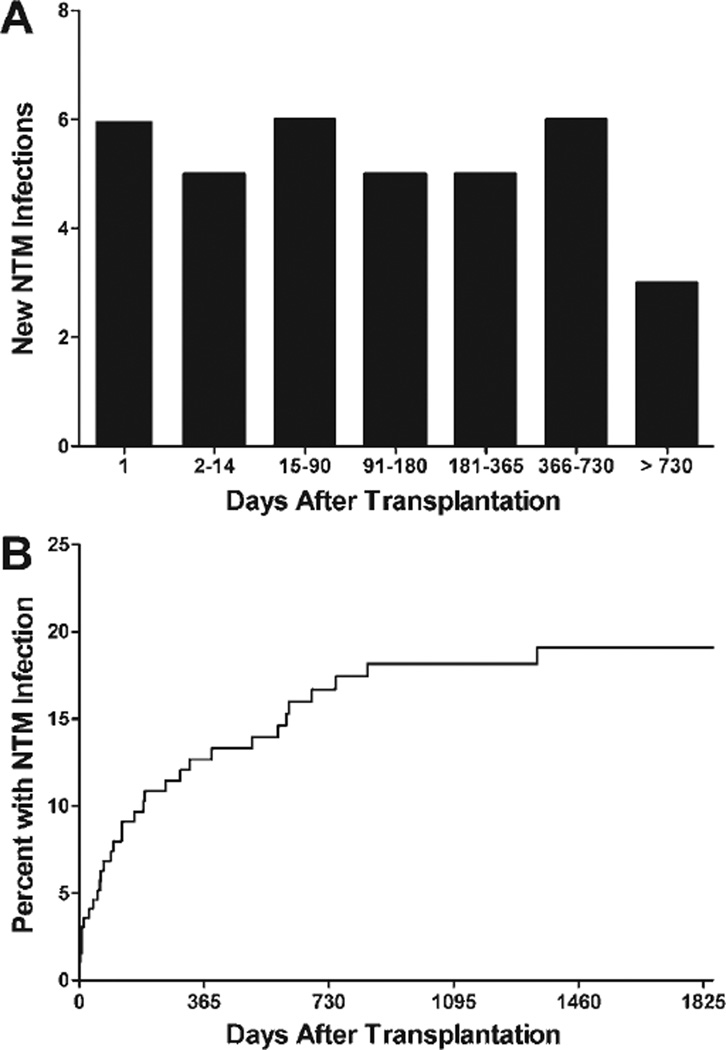
Among those recipients diagnosed with NTM infection, the median time to initial NTM isolation was 97 days (IQR 8 to 371 days) (Figure 1). The median time to a diagnosis of NTM disease was 583 days (IQR 203 to 735 days). NTM colonization was diagnosed prior to NTM disease in 4 recipients, by an average 300 days (range 75 to 536 days). Only 5 cases of NTM infection, 2 of which were NTM disease, were first diagnosed after a diagnosis of BOS.
Figure 1.
The timing (A) and cumulative incidence (B) of NTM infections in lung transplant recipients.
Among baseline characteristics, only single lung transplant was a predictor of NTM infection in Cox proportional hazards models (hazard ratio [HR] 2.25, 95% confidence interval [CI] 1.11 to 4.58, p = 0.02). Single lung transplant was not associated with NTM disease. When considered as time-dependent variables, NTM colonization was a significant risk factor for NTM disease (HR 8.39, 95% CI 2.08 to 33.85, p = 0.003). The first treated AR episode trended, but was not a significant risk factor for NTM disease (HR 3.71, 95% CI 0.88 to 15.63, p = 0.07). The small number of NTM disease cases prevented multivariate analyses.
NTM infection is a predictor of mortality
When considered as a time-dependent variable, the development of NTM infection post-transplantation was associated with a significantly increased risk of mortality (unadjusted HR 2.61, 95% CI 1.59 to 4.28, p = 0.001) (Table 4). To further explore this relationship, we considered NTM colonization and NTM disease separately. A similar increased hazard for mortality was observed for both NTM colonization and NTM disease, although the risk for death tended to be greatest among patients who developed NTM disease (Table 4). We also sought to confirm the impact of NTM infection on mortality in multivariate models adjusted for single lung transplant and BOS (time-dependent). As shown in Table 4, NTM infection, NTM colonization and NTM disease each remained a significant predictor of mortality after adjustment for single lung transplant and for BOS.
Table 4.
Association of NTM and Mortality: Univariate and Multivariate Models
| Univariate models | Multivariate modelsa | |||
|---|---|---|---|---|
| NTM variable type | HR (95% CI) | p | HR (95% CI) | p |
| NTM infection | 2.61 (1.59–4.28) | 0.001 | 2.18 (1.26–3.76) | 0.005 |
| NTM colonization | 2.47 (1.42–4.31) | 0.002 | 1.93 (1.03–3.62) | 0.04 |
| NTM disease | 3.98 (1.69–9.37) | 0.002 | 3.50 (1.46–8.38) | 0.005 |
CI, confidence interval; HR, hazard ratio; NTM, non-tuberculous mycobacterium.
Adjusted for single lung transplant (time-independent) and bronchiolitis obliterans syndrome (time-dependent).
Given the greater number of bronchoscopies performed in the NTM disease group (Table 1), we explored the effect of a potential sampling bias on the risk for mortality. The number of bronchoscopies performed was not associated with mortality (HR 0.98, 95% CI 0.91 to 1.06, p = 0.70).
NTM infection and the risk of BOS
We considered NTM infection as a time-dependent variable in similar Cox models for BOS (Table 5). Although the hazard for BOS tended to be increased in patients with NTM infection, the association was not statistically significant. When considered separately, neither NTM colonization nor NTM disease was significantly associated with an increased risk of BOS. After adjustment for single lung transplant and the cumulative AR score, the hazard for BOS associated with each NTM variable increased, but without statistical significance (Table 5).
Table 5.
Association of NTM and BOS: Univariate and Multivariate Models
| Univariate models | Multivariate modelsa | |||
|---|---|---|---|---|
| NTM variable type | HR (95% CI) | p | HR (95% CI) | p |
| NTM infection | 1.57 (0.87–2.82) | 0.13 | 1.77 (0.97–3.25) | 0.06 |
| NTM colonization | 1.33 (0.65–2.69) | 0.44 | 1.54 (0.75–3.20) | 0.24 |
| NTM disease | 2.32 (0.93–5.79) | 0.07 | 2.37 (0.94–5.96) | 0.07 |
BOS, bronchiolitis obliterans syndrome; CI, confidence interval; HR, hazard ratio; NTM, non-tuberculous mycobacterium.
Adjusted for single lung transplant (time-independent) and cumulative AR score (time-independent).
Causes of death
In seeking a possible explanation for the increased mortality associated with NTM, we determined the cause of death from the medical records. There were 84 deaths (42%) in the total cohort recorded during the follow-up period: 62 (38%) in the non-NTM group and 22 (61%) in the NTM infection group. NTM disease was never determined to be the direct cause of mortality in our study. BOS was determined to be a contributing factor in 26 (42%) deaths in the non-NTM group and 14 (64%) deaths in the NTM group, but this difference was not statistically different (p = 0.08). However, non-NTM infection was a significantly more common contributing cause of death in the NTM group, contributing to 12 (19%) deaths in the non-NTM group and 9 (41%) deaths in the NTM group (p = 0.04).
Discussion
Infections and BOS are the two most important factors limiting long-term survival post-transplant. Data regarding NTM infection complicating lung transplantation are sparse and limited to only one series comprising 23 cases,23 a few reviews19,21,22 and isolated case reports.18,20,24–28 The role of NTM in mortality has not been appreciated. We hypothesized that NTM infection after lung transplantation may be an independent risk factor for increased mortality after lung transplantation.
In our cohort, NTM infections occurred in 18% of lung transplant recipients, a rate slightly higher than previous reports of 4% to 9%.22,23 This difference may be attributable to the advancement in methodology in the mycobacteriology laboratory, resulting in enhanced isolation and more rapid and accurate identification of NTM from clinical specimens.29
We found that single lung transplant was associated with post-transplant NTM infection. Single lung transplant was not associated with NTM disease, but the small number of NTM disease patients limits the conclusions that can be drawn from this finding. Prior studies in non-transplant patients have demonstrated that lung structural abnormalities are associated with NTM infection.32 In single lung transplant recipients, the native abnormal lung may harbor NTM at the time of transplantation, or it may facilitate de novo NTM infections after transplantation. Although cystic fibrosis is a risk factor for NTM, we did not find an association between cystic fibrosis and post-transplant NTM infections, and this is consistent with a prior study.33 All cystic fibrosis patients receive a bilateral lung transplant, and this may partially explain the absence of increased risk for post-transplant NTM infections. We acknowledge that our cohort consisted of a very small number of cystic fibrosis patients.
It is not surprising, but noteworthy, that NTM colonization was a risk factor for NTM disease in our study. According to the damage–response framework of microbial pathogenesis, colonization and disease states are generally continuous over time, such that when damage caused by an infection exceeds a threshold the disease becomes evident.34 Moreover, in some instances, where the host response is weakened, the rate of damage is accelerated. In this study, the risk for developing NTM disease was increased after treated acute rejection (augmented immune suppression), but this trend was not statistically significant. Nevertheless, an increased risk of NTM disease after AR treatment would be consistent with the damage–response framework of microbial pathogenesis.
To our knowledge, this is the first study demonstrating that post-transplant NTM infection is associated with a higher risk for mortality. No recipients died directly from NTM, a finding corroborated by other studies.22,23 Furthermore, even NTM colonization was a risk factor for mortality. It is likely that NTM infection in lung transplant recipients is a marker for structural defects in the lungs. However, the elevated risk for mortality persisted in both NTM colonization and NTM disease even after adjusting for single lung transplantation and BOS. We speculate that, for some patients, NTM infection after lung transplantation is a marker for a general impaired ability to mount an effective immune response to infection and may suggest excessive immunosuppression. In support of this idea, we found that the NTM infection group had a higher mortality rate attributable to non-NTM infectious diseases when compared with the non-NTM group.
Based on recent studies demonstrating that fungal, bacterial and viral infections increase the risk of post–lung transplant BOS,2–16 another plausible explanation for increased mortality could be that NTM increases the risk of BOS. We did find an increased hazard for BOS with NTM infection, but this association did not reach statistical significance. Moreover, our multivariate models of mortality were adjusted for BOS. Ultimately, a larger cohort will be required to determine whether NTM is a risk factor for BOS.
Our study has several potential limitations. First, as is inherent to any retrospective study, incomplete or missing data limit our findings. As a specific example, during the period of this study, there were no standard protocols for NTM screening of potential lung transplant recipients or of donor lungs. These studies were inconsistently performed in our cohort. Second, unique aspects of our single-center cohort may limit generalization to all lung transplant recipients. Our center transplants a disproportionately high percentage of patients with restrictive lung disease and a small percentage of patients with cystic fibrosis. Third, our relatively small sample size did not permit extensive adjustment for other mortality risk factors without overburdening our models. A larger, multicenter cohort would be better suited to dissect the indirect effects of NTM infection on mortality and potentially on BOS. Further, uncontrolled studies have suggested that macrolide therapy may be effective in treating some patients with BOS.35–38 A macrolide antibiotic is commonly included in treatment regimens for NTM. We attempted to discern whether macrolide therapy for NTM infection had a beneficial effect on mortality (data not shown). However, the use of a macrolide in our cohort correlated strongly with the presence of NTM disease. Given the potential for multicollinearity in the presence of NTM disease, we are unable to differentiate between the impacts of macrolide therapy on any long-term outcomes independent of the impact of NTM disease.
We are uncertain whether or to what degree NTM infection is a modifiable risk factor for poor outcomes after transplantation. One would expect that identification and treatment of pre-transplant NTM infection would reduce the incidence of post-transplant NTM infections. We did not find an increased risk of NTM infection or disease in recipients or donor lungs that screened positive for NTM infection. Presumably, the absence of an increased risk of post-transplant NTM infection in these patients was related to treatment received before and after transplantation. At least one recipient of a single lung who was not screened pre-transplant was found to have native lung NTM disease at the time of transplant. A rigorous pre-transplant screening for NTM infection may identify more patients to be treated pre-transplant and reduce post-transplant infections. We do not believe it is practical to perform bronchoscopy on all potential lung transplant recipients, but in patients who produce sputum we routinely obtain sputum cultures to screen for mycobacterium and other infections. It is now our practice to exclude potential recipients with NTM infection from listing until completion of at least 3 months of therapy.
Although AR treatment may be a risk factor for NTM disease, the potential risks of NTM prophylaxis with every episode of AR likely outweigh the benefit of preventing a rare case of NTM disease. In the case of an NTM-colonized patient who develops AR, NTM suppressive therapy may be warranted, although this was not tested in our study. Among patients who develop post-transplant NTM colonization, we attempt to reduce immunosuppression when feasible. We also typically treat colonization with M abscessus and consider treatment in cases of recurrent isolation of any other NTM species. We always treat NTM disease. Antibiotics are selected based on NTM species, sensitivities, expected toxicities and interactions with immunosuppressive agents. The duration of treatment depends on the clinical course, but in the case of NTM disease the therapy is prolonged and followed by lifelong suppressive therapy when appropriate.
In conclusion, we have demonstrated that NTM infection is a risk factor for mortality after lung transplantation. Importantly, this effect appears to be independent of the impact of BOS on mortality. The mechanisms underlying this association are unclear, but we speculate that NTM infection may be a marker for increased susceptibility to non-NTM infections that may directly impact survival. Future investigations are required to validate our findings and to better elucidate the mechanism(s) involved.
Acknowledgments
This study was supported in part by the National Institutes of Health (HL 080206 to J.A.B. and HL 094746 to S.S.W.).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart–lung transplant report—2010. J Heart Lung Transplant. 2010;29:1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85:771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 3.Glanville AR, Gencay M, Tamm M, et al. Chlamydia pneumoniae infection after lung transplantation. J Heart Lung Transplant. 2005;24:131–136. doi: 10.1016/j.healun.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 4.Heng D, Sharples LD, McNeil K, et al. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–1263. [PubMed] [Google Scholar]
- 5.Keenan RJ, Lega ME, Dummer JS, et al. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51:433–438. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Keller CA, Cagle PT, Brown RW, et al. Bronchiolitis obliterans in recipients of single, double, and heart–lung transplantation. Chest. 1995;107:973–980. doi: 10.1378/chest.107.4.973. [DOI] [PubMed] [Google Scholar]
- 7.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 8.Kotsimbos TC, Snell GI, Levvey B, et al. Chlamydia pneumoniae serology in donors and recipients and the risk of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2005;79:269–275. doi: 10.1097/01.tp.0000149839.87843.64. [DOI] [PubMed] [Google Scholar]
- 9.Kroshus TJ, Kshettry VR, Savik K, et al. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114:195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 10.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neurohr C, Huppmann P, Leuchte H, et al. Human herpesvirus 6 in bronchalveolar lavage fluid after lung transplantation: a risk factor for bronchiolitis obliterans syndrome? Am J Transplant. 2005;5:2982–2991. doi: 10.1111/j.1600-6143.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 12.Reichenspurner H, Girgis RE, Robbins RC, et al. Stanford experience with obliterative bronchiolitis after lung and heart–lung transplantation. Ann Thorac Surg. 1996;62:1467–1472. doi: 10.1016/0003-4975(96)00776-X. [DOI] [PubMed] [Google Scholar]
- 13.Smith MA, Sundaresan S, Mohanakumar T, et al. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116:812–820. doi: 10.1016/S0022-5223(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 14.Vos R, Vanaudenaerde BM, Geudens N, et al. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J. 2008;31:1037–1045. doi: 10.1183/09031936.00128607. [DOI] [PubMed] [Google Scholar]
- 15.Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9:1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigt SS, Elashoff RM, Keane MP, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant. 2008;8:1512–1522. doi: 10.1111/j.1600-6143.2008.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentine VG, Gupta MR, Walker JE, Jr, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2009;28:163–169. doi: 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 18.Baldi S, Rapellino M, Ruffini E, et al. Atypical mycobacteriosis in a lung transplant recipient. Eur Respir J. 1997;10:952–954. [PubMed] [Google Scholar]
- 19.Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis. 2004;38:1428–1439. doi: 10.1086/420746. [DOI] [PubMed] [Google Scholar]
- 20.Fairhurst RM, Kubak BM, Shpiner RB, et al. Mycobacterium abscessus empyema in a lung transplant recipient. J Heart Lung Transplant. 2002;21:391–394. doi: 10.1016/s1053-2498(01)00339-4. [DOI] [PubMed] [Google Scholar]
- 21.Flume PA, Egan TM, Paradowski LJ, et al. Infectious complications of lung transplantation. Impact of cystic fibrosis. Am J Respir Crit Care Med. 1994;149:1601–1607. doi: 10.1164/ajrccm.149.6.7516251. [DOI] [PubMed] [Google Scholar]
- 22.Kesten S, Chaparro C. Mycobacterial infections in lung transplant recipients. Chest. 1999;115:741–745. doi: 10.1378/chest.115.3.741. [DOI] [PubMed] [Google Scholar]
- 23.Malouf MA, Glanville AR. The spectrum of mycobacterial infection after lung transplantation. Am J Respir Crit Care Med. 1999;160:1611–1616. doi: 10.1164/ajrccm.160.5.9808113. [DOI] [PubMed] [Google Scholar]
- 24.Sanguinetti M, Ardito F, Fiscarelli E, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol. 2001;39:816–819. doi: 10.1128/JCM.39.2.816-819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swetter SM, Kindel SE, Smoller BR. Cutaneous nodules of Mycobacterium chelonae in an immunosuppressed patient with preexisting pulmonary colonization. J Am Acad Dermatol. 1993;28:352–355. doi: 10.1016/0190-9622(93)70053-v. [DOI] [PubMed] [Google Scholar]
- 26.Torres F, Hodges T, Zamora MR. Mycobacterium marinum infection in a lung transplant recipient. J Heart Lung Transplant. 2001;20:486–489. doi: 10.1016/s1053-2498(00)00185-6. [DOI] [PubMed] [Google Scholar]
- 27.Trulock EP, Bolman RM, Genton R. Pulmonary disease caused by Mycobacterium chelonae in a heart–lung transplant recipient with obliterative bronchiolitis. Am Rev Respir Dis. 1989;140:802–805. doi: 10.1164/ajrccm/140.3.802. [DOI] [PubMed] [Google Scholar]
- 28.Woo MS, Downey S, Inderlied CB, et al. Pediatric transplant grand rounds. A case presentation: skin lesions in a post–lung transplant patient. Pediatr Transplant. 1997;1:163–170. [PubMed] [Google Scholar]
- 29.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 30.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 31.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 32.Griffith DE, Girard WM, Wallace RJ., Jr Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 33.Bonvillain RW, Valentine VG, Lombard G, et al. Post-operative infections in cystic fibrosis and non-cystic fibrosis patients after lung transplantation. J Heart Lung Transplant. 2007;26:890–897. doi: 10.1016/j.healun.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Casadevall A, Pirofski LA. The damage–response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerhardt SG, McDyer JF, Girgis RE, et al. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168:121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb J, Szangolies J, Koehnlein T, et al. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85:36–41. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 37.Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;77:1465–1467. doi: 10.1097/01.tp.0000122412.80864.43. [DOI] [PubMed] [Google Scholar]
- 38.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172:772–775. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]