Figure 6.
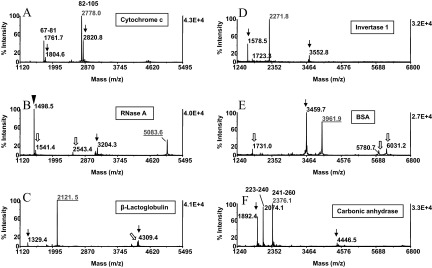
CNBr cleavage of model proteins. Proteins (50 pmoles) were reduced, carboxyamidomethylated, and subjected to CNBr cleavage in the one-pot reaction scheme. The reaction mixture was absorbed onto ZipTipC18 pipette tips, which were desalted and eluted. The eluates were analyzed by MALDI-MS. The MALDI-MS spectra were annotated with the individual proteins examined. Masses highlighted in red designate the C-terminal CNBr fragments identified by database search. Fragments recognized as proximal to each other in the protein sequences are annotated with the starting and ending amino acids. Arrows and open arrows denote homoserine lactone-terminated peptides, recognized as truncation products and internal, unspecific degradation products, respectively (see text for details). Filled arrowhead indicates the protein's N-terminal CNBr fragment. One-tenth of the eluates was applied to the target. Note that C-terminal fragments, harboring cysteinyl residues, were recovered in their fully carboxyamidomethylated form (B, C, and E). The sequences of the CNBr C-terminal fragments ascertained by database search are listed in Table 1. One-tenth of the eluates was applied to the target.
