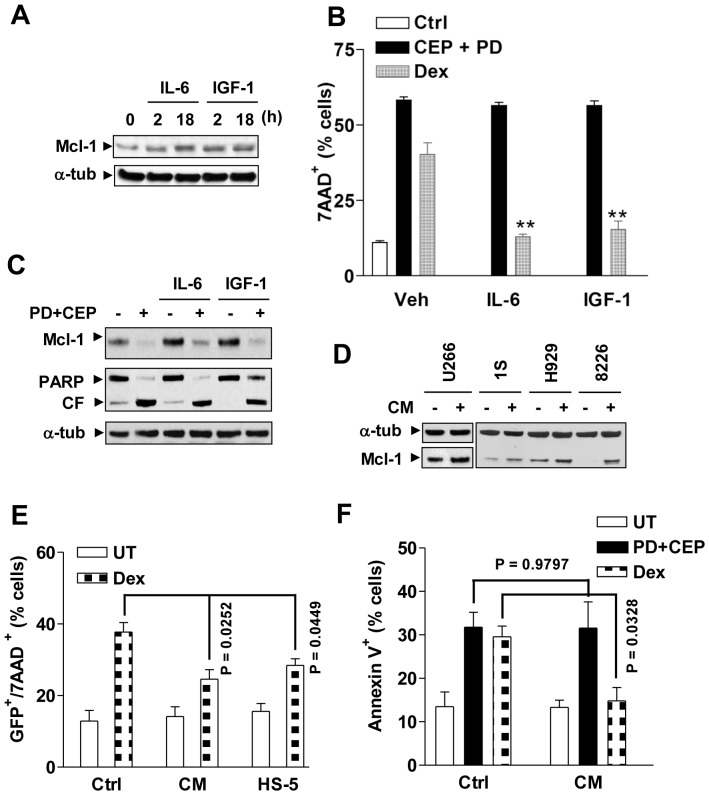
Figure 4. PD184352/CEP3891 attenuates Mcl-1 up-regulation and drug-resistance induced by growth factors.
(A) U266 cells were cultured in serum-free medium for 6 h, followed by addition of IL-6 (100 ng/ml) or IGF-1 (400 ng/ml) for 2 h and 18 h, after which cells were lysed and subjected to Western blot analysis to assess expression of Mcl-1. (B) U266 cells were treated with 400 nM CEP3891+7.5 µM PD184352 or 50 µM dexamathasone (Dex) for 40 h in either the presence or absence of IL-6 or IGF-1. After treatment, the percentage of dead cells was evaluated by flow cytometry (** P<0.01 vs. without IL-6 or IGF-1). (C) After pre-incubation with either IL-6 or IGF-1 for 1 h, U266 cells were exposed to 400 nM CEP3891+5 µM PD184352 for 4 h, after which Western blot analysis was performed to monitor Mcl-1 expression and PARP cleavage. (D) MM.1S, H929, RPMI8226, and U266 cells were serum-starved for 6 h, and then cultured for an additional 24 h in either fresh 10% FBS medium as a control or conditioned medium (CM) derived from HS-5 cell cultures, after which Western blot analysis cells was performed to assess Mcl-1 expression. (E) U266 cells stably expressing GFP were pre-cultured with HS-5 cells or in the presence of HS-5 CM for 48 h, followed by treatment with 50 µM dexamathasone for an additional 40 h. After treatment, the percentage of dead (7AAD+) cells in the GFP+ population was determined by flow cytometry. (F) H929 cells were exposed to 6 µM dexamathasone or 400 nM CEP3891+2.5 µM PD184352 for 38 h in the presence of HS-5 CM, after which the percentage of apoptotic (Annexin V+) cells was determined by flow cytometry. For panels 4E and F, Ctrl = 10% FBS medium. For flow cytometry, values represent the means ± SD for three separate experiments.