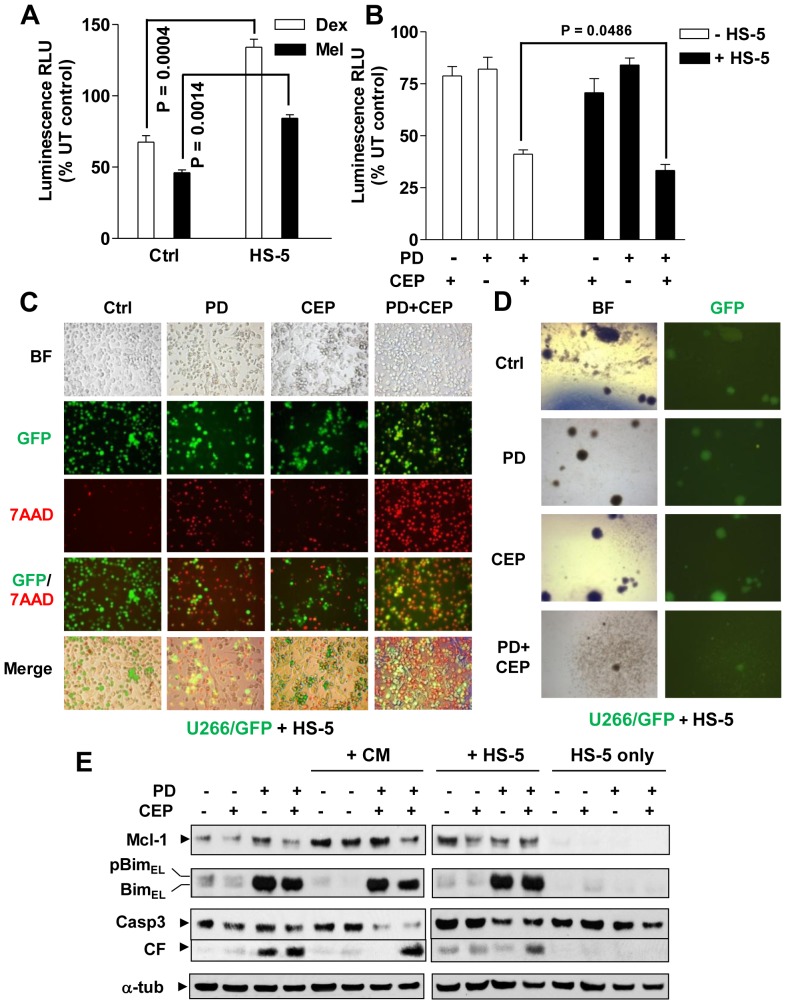
Figure 5. BMSCs fail to protect MM cells from PD184352/CEP3891 lethality.
(A) and (B) U226 cells stably expressing luciferase were co-cultured for 24 h with HS-5 cells (pre-cultured for 48 h), and then treated with either 50 µM dexamethasone (Dex) or 30 µM melphalan (Mel, A) or 400 nM CEP3891±7.5 µM PD184352 (B) for an additional 48 h. Bioluminescence intensity, which is proportional to the number of living cells, was monitored to assess cell viability. Values represent the means and SD for three separate experiments performed in triplicate. UT = untreated; RLU = relative light unit. (C) GFP-expressing U266 cells were co-cultured for 48 h with HS-5 cells (pre-cultured for 48 h) on the 4-well chamber slides, after which cells were treated with 400 nM CEP3891±7.5 µM PD184352 for an additional 40 h. Cells were then stained with 7AAD and images captured by an inverted fluorescence microscope (Olympus 1X71, 20× objective) with the filters suitable for 7AAD (red) or GFP (green). In parallel, bright field (BF) images were also captured for the same areas. (D) After treatment as described in panel 5B, GFP-expressing U266 cells were washed free of drugs and then plated with HS-5 cells on soft agar. After incubation for 21 days, the colony-forming ability of GFP+ U266 cells was assessed under fluorescence microscopy (Olympus 1X71, 4× objective); colonies were defined as clusters of >50 GFP+ cells. Bright field images were captured for comparison. The microscopic images are representative of three separate experiments. (E) H929 cells were treated with 300 nM CEP3891±2.5 µM PD184352 for 48 h under the conditions as follows: a) 10% FBS medium as control (lanes 1–4); b) HS-5-derived conditional medium (CM, lanes 5–8); and c) co-culture with HS-5 (lanes 9–12). In parallel, HS-5 cells alone (lanes 13–16) were treated for comparison. After drug treatment, Western blot analysis was conducted to monitor the expression of Mcl-1 and Bim, as well as caspase 3 cleavage.