Figure 5.
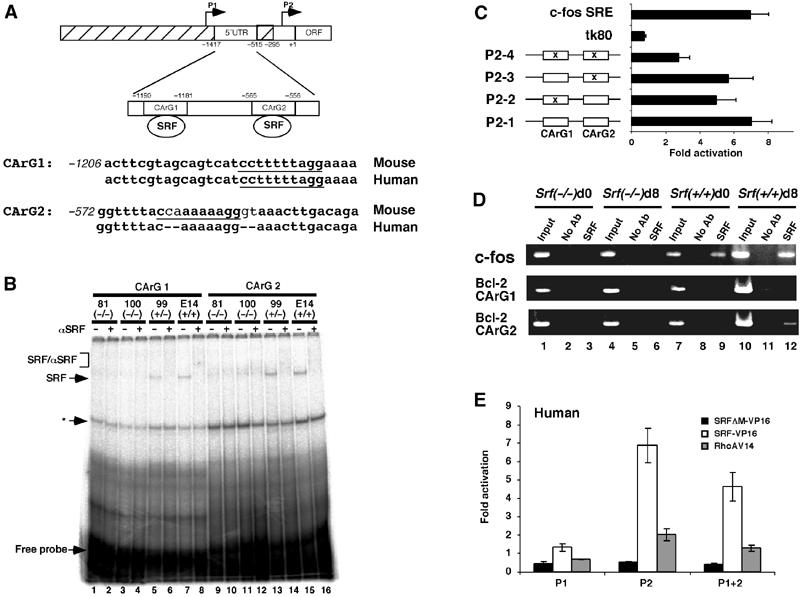
SRF binds directly to the murine Bcl-2 regulatory region in vitro and in vivo and activates Bcl-2 P2 promoter-driven reporter gene transcription. (A) Schematic of the murine Bcl-2 gene regulatory region. Transcriptional start sites under the control of the P1 and P2 regions are indicated by arrows. Nucleotide coordinates are oriented with regard to the beginning of the murine Bcl-2 ORF (+1). The two conserved CArG box sequences lie within a 5′-untranslated exon. Intronic sequences are hatched. Murine and human genomic sequence comparison of the CArG1- and CArG2-containing DNA segments is given. (B) EMSA analysis of Bcl-2 CArG box binding activity in ES cells of different Srf genotype. In all, 15 μg of total ES cell extract was incubated with CArG 1 (lanes 1–8) or CArG 2 (lanes 9–16) oligonucleotides in the presence or absence of anti-SRF antibody (α-SRF). SRF: SRF/DNA complex; SRF/αSRF: SRF/α-SRF/DNA complex. Asterisk (*) denotes an unidentified complex that is not supershifted by an anti-SRF antibody. (C) SRF-VP16 activates the murine Bcl-2 P2 promoter in a CArG box-dependent manner. P2-1 to -4, tk80-luc, and c-fos SRE-luc were transfected into NIH3T3 along with an SRF-VP16 expression vector. Conserved CArG box motives are indicated by open boxes, and CArG box mutations are marked by x. Fold activation over empty vector is given, representing the mean of three independent experiments ±s.d. (D) ChIP analysis of SRF binding to the genomic c-fos and Bcl-2 CArG elements. PCR was performed on immunoprecipitated chromatin fragments of 100 Srf(−/−) (lanes 1–6) or wt (lanes 7–12) ES cells, either undifferentiated (d0; lanes 1–3 and 7–9) or in vitro differentiated for 8 days (d8; lanes 4–6 and 10–12). Lanes 1, 4, 7, and 10 show amplification of total input DNA (1:100 dilution). Lanes 2, 5, 8, and 11 show amplification of ChIP reactions with no antibody. Lanes 3, 6, 9, and 12 show amplification of target sequences in ChIP reactions with anti-SRF antibody. (E) NIH3T3 cells were transfected with the luciferase reporter gene constructs driven by human Bcl-2 promoters (P1-luc, P2-luc, P1+2-luc) along with either empty vector or expression vectors encoding for SRF-VP16, SRFΔM-VP16, or constitutively active RhoA-V14. Normalized luciferase activity and fold activation was derived as in (C). Values represent the mean of three independent experiments ±s.d.
