Figure 1.
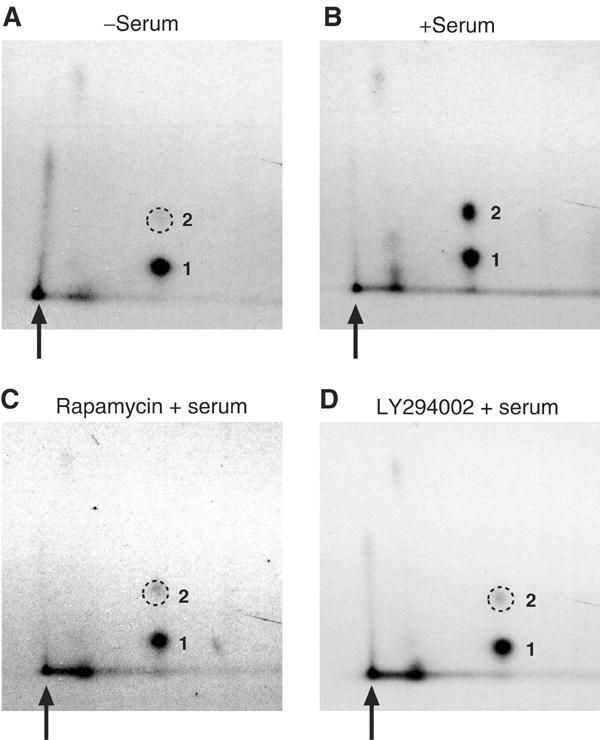
Phosphorylation of a single major eIF4B peptide is modulated by serum, and is sensitive to pharmacological inhibitors of PI3K and mTOR. Phosphopeptide mapping of endogenous eIF4B isolated from 293 cells, (A) starved of serum for 36 h; (B) starved of serum, and then serum stimulated for 30 min; (C) starved of serum, pretreated with 5 μM LY294002 for 30 min, and then serum stimulated for 30 min; or (D) starved of serum, pretreated with 50 nM rapamycin for 30 min, and then serum stimulated for 30 min. The two major phosphopeptides are numbered, and the loading origin is indicated by an arrow. The position of the diminished phosphopeptide 2 is indicated with a dashed circle. A vertical streaked signal near the loading origin is also observed, but this signal does not appear to be responsive to serum stimulation nor sensitive to kinase inhibitors.
