Figure 5.
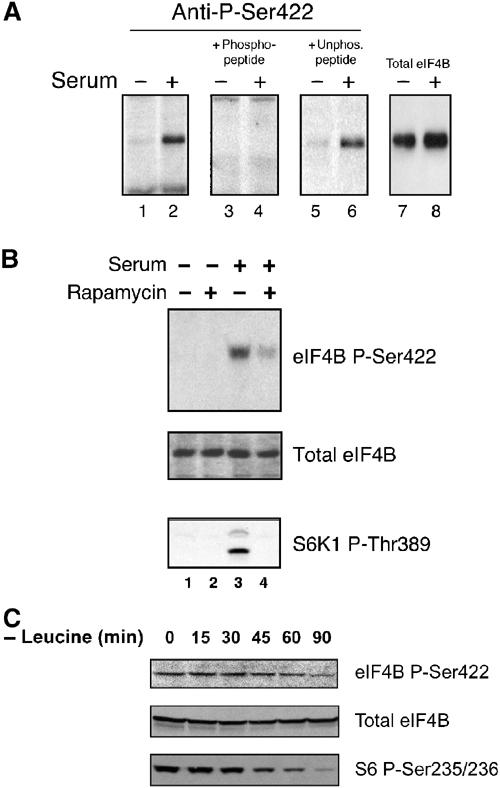
A phosphospecific antiserum confirms that phosphorylation of Ser422 is serum responsive, and is sensitive to rapamycin treatment and leucine deprivation. (A) Cells were starved of serum (odd-numbered lanes), or starved of serum and then serum stimulated (even-numbered lanes). Lysates were subjected to Western analysis using the phosphospecific eIF4B Ser422 antiserum (lanes 1 and 2). Preincubation of the antiserum with the phosphopeptide against which it was raised (lanes 3 and 4), or with the same peptide containing an unphosphorylated Ser422 (lanes 5 and 6). eIF4B expression levels were not affected by these treatments (lanes 7 and 8). (B) Cells were pretreated with rapamycin, and subjected to Western analysis using the phosphospecific antiserum (top panel) and antisera directed against total eIF4B (middle panel), as above. The same lysates were subjected to Western analysis using a phosphospecific antibody directed against S6K1 Thr389 (lower panel). (C) Cells were subjected to leucine-free (but otherwise complete) culture media for the indicated time periods. Lysates were subjected to Western blotting using the eIF4B Ser422 phosphospecific antiserum (top panel), a phosphorylation-independent antiserum directed against eIF4B (middle panel) or a phosphospecific antiserum directed against ribosomal S6 protein Ser235/236 (lower panel).
