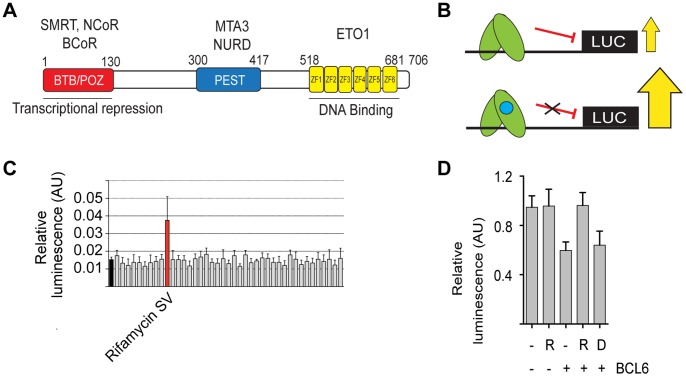
Figure 1. A natural product screen to identify novel inhibitors of BCL6 transcriptional repression.
(A) Schematic of BCL6 showing amino-terminal POZ domain (red), carboxy terminal zinc fingers (yellow) and mid portion containing PEST domains (blue). Different proteins associate with the three portions of BCL6. NCoR, BCoR and SMRT associate with the POZ domain, MTA3 and NuRD with the mid portion and ETO1 with the zinc fingers. (B) Illustration of the screening strategy. BCL6 (green) is shown associating with its binding site cloned upstream of a luciferase reporter gene. Without any compound, or with an inactive compound i.e. one that does not bind BCL6, luciferase output is repressed but in the presence of active compound BCL6 mediated repression is prevented and output of luciferase increases. (C) Screening results for half a plate (40 compounds) from the natural product library. The black bar (furthest left) is the mean negative control i.e. transfected cells without test compound, and the black horizontal line the mean value across the entire screen. The red bar shows rifamycin SV. (D) The effect of rifamycin is due to inhibition of BCL6 transcriptional repression. HEK293T cells were co-transfected with a BCL6 expression construct and a luciferase reporter. Transcriptional repression due to BCL6 was relieved by rifamycin SV (R), but not by an agent that was ineffective in the screen (D).