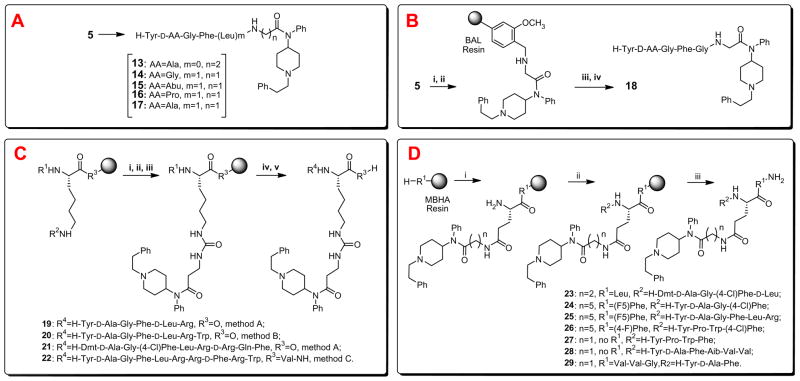
Scheme 2.
Our strategies to introduce 4-anilidopiperidine functionality to opioid ligands. Reagents and conditions: (A) stepwise solution phase peptide synthesis, analogs 13–17; (B) reductive alkylation on solid support using (i) 4-(4-formyl-3-methoxy-phenoxy)butyryl AM (BAL) resin, NaBH(OAc)3, DMF/DCM (1/3), (ii) Fmoc-AA-OH, HOBt, HBTU, DIPEA in DMF, (iii) 50% Piperidine in DMF, and (iv) TFA/i-PrSiH3/H2O (90/5/5); (C) N-side chain modification by method A: Merrifield resin (R3=O, R1 = Boc, R2 = Fmoc), (i) 50% Piperidine in DMF, (ii) p-nitrochloroformate, DIPEA, DCM, 1 h, (iii) 2–3 equiv of 3-aminofentanyl, microwave in a closed vessel for 1 h at 80 °C, (iv) 50% TFA in DCM, (v) SPPS followed by TFMSA cleavage; method B: 2-chlorotrityl resin (R3=O, R1 = Fmoc, R2 = Alloc), (i) Pd(PPh3)4, PhSiH3 in DCM, (ii) p-nitrochloroformate, DIPEA, DCM, 1 h, (iii) 2–3 equiv of 3-aminofentanyl, microwave in a closed vessel for 1 h at 80 °C, (iv) 50% piperidine in DMF, (v) SPPS followed by TFA cleavage; method C: MBHA resin (R3 = Val-NH, R1 = Fmoc, R2 = Boc), (i) 50% TFA in DCM, (ii) p-nitrochloroformate, DIPEA, DCM, 1 h, (iii) 2–3 equiv of 3-aminofentanyl, microwave in a closed vessel for 1 h at 80 °C, (iv) 50% piperidine in DMF, (v) SPPS followed by TFMSA cleavage; (D) C-side chain modification using (i) Fmoc-Glu(Boc)-OH, HOBt, DIC, DMF→50% TFA in DCM→5% DIEA in DCM→amine (1, 5, 3-aminofentanyl), HOBt, DIC, microwave in DMF for 5 min at 80 °C→25% piperidine in DMF, (ii) solid phase peptide synthesis, and (iii) TFMSA cleavage.