Figure 1.
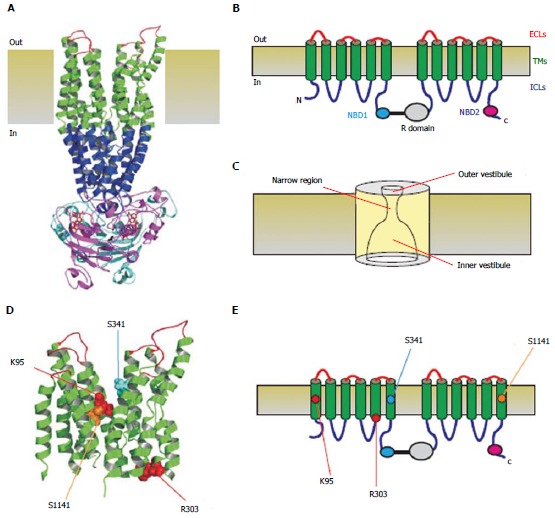
Three-dimensional and two-dimensional representations of cystic fibrosis transmembrane conductance regulator structure. A: Atomic homology model of cystic fibrosis transmembrane conductance regulator in a so-called “channel like” conformation[20]. Different colours are used to illustrate the approximate extent of the extracellular loops (ECLs, red), transmembrane domains (TMs, green), intracellular loops (ICLs, blue), and two nucleotide binding domains (NBD1, cyan; NBD2, magenta). The cytoplasmic R domain is not included in this homology model; B: Schematic representation of these different domains (and the R domain), using the same colour scheme; C: Functional model of pore architecture. As described in the text, experimental evidence suggests that the pore has a narrow region that is connected to the cytoplasmic and extracellular solutions by a wide inner vestibule and a narrower outer vestibule, respectively; D: Location of putative blocker-interacting residues in the TMs (K95-TM1; R303-TM5; S341-TM6; S1141-TM12) within the same homology model shown in A. E: Location of these same residues in the same schematic model shown in B.
