Figure 2.
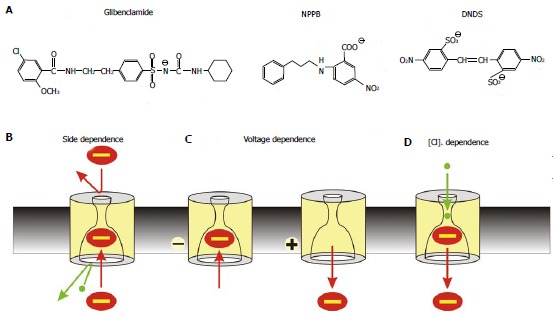
Mechanism of open channel blocker action. A: Chemical structures of three well-known voltage-dependent cystic fibrosis transmembrane conductance regulator (CFTR) channel blockers: the sulfonylurea glibenclamide, the aryl amino benzoate 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), and the disulfonic stilbene 4,4’-dinitrostilbene-2,2’-disulfonic acid (DNDS). B-D: Characteristic functional properties of block shared by these and other CFTR open channel blockers: block is side-dependent, voltage-dependent, and sensitive to extracellular Cl- concentration; B: Blockers enter the pore only from its cytoplasmic end, likely because the extracellular entrance to the pore is too small and/or the narrow pore region prevents them from accessing their binding site in the wide inner vestibule; C: Block is relatively strengthened at hyperpolarized membrane potentials that favour entry of negative substances into the pore from the cytoplasm, and weakened at depolarized membrane potentials that favour anion retention in the cytoplasm; D: Block is weakened at higher extracellular Cl- concentrations; this is usually ascribed to a “knock-off” mechanism whereby Cl- entering the pore from its extracellular end electrostatically repels negatively charged blockers back into the cytoplasm, destabilizing blocker binding inside the pore.
