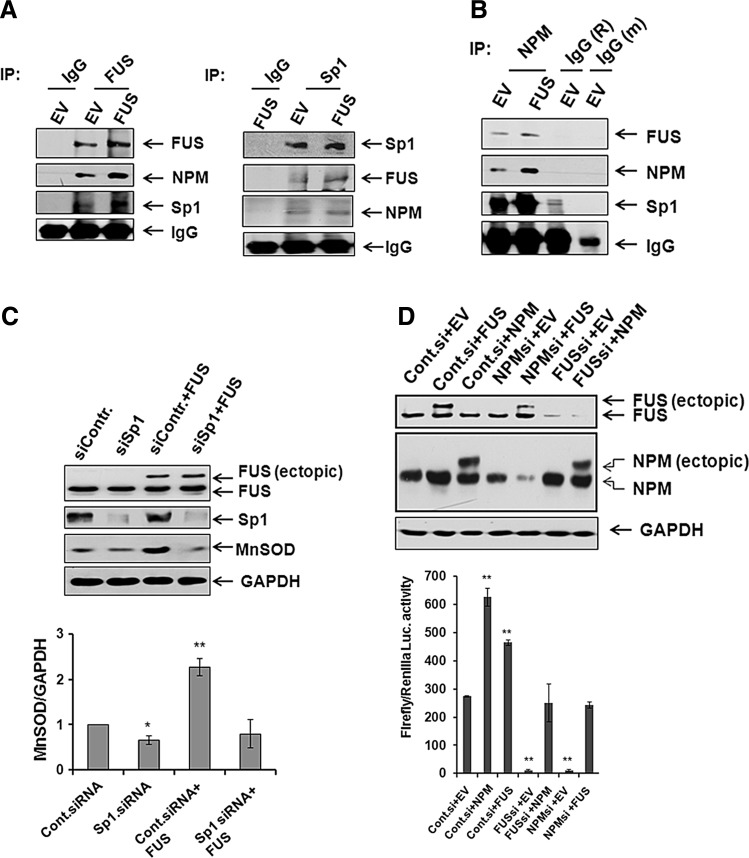
FIG. 7.
Association of FUS with NPM and specificity protein 1 (Sp1). In vivo association of FUS with NPM and Sp1 in isolated nuclear extract was detected by coimmunoprecipitation using FUS or Sp1 antibody. Coimmunoprecipitation by anti-rabbit IgG from nuclear extract was used as negative control (A). Reverse immunoprecipitation was also carried out using NPM antibody, and FUS and Sp1 were detected in the immunocomplex by Western blotting (B). Cells were transfected with Sp1 siRNA or control siRNA with or without FUS expression vector for 48 h. After the transfection, cell lysates were prepared and subjected to SDS-polyacrylamide gel electrophoresis (C). FUS, Sp1, and MnSOD proteins were detected by Western blotting using antibodies specific to FUS, Sp1, and MnSOD, respectively. GAPDH was used as loading control (top panel). MnSOD protein bands were densitometrically scanned and quantified using GAPDH as loading control (bottom panel). MnSOD promoter driven luciferase activity was measured after transfection of combined NPM siRNA and FUS expression vector or a combination of FUS siRNA and NPM expression vector in JB6 cells (D, bottom panel). The suppression of proteins after transfection of corresponding siRNA and overexpression of proteins after transfection of corresponding expression vector were confirmed by Western blotting (D, upper panel). Each data point represents the mean±SD of three independent experiments. Significant difference as compared with corresponding control is indicated by *p<0.05 and **p<0.01.