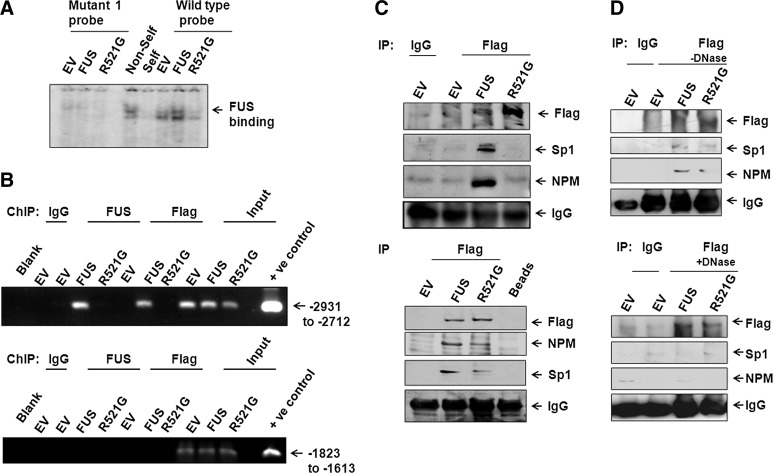
FIG. 8.
Mutation in FUS abrogates its activity for MnSOD promoter binding and protein-protein interaction. EMSA was carried out with nuclear extracts from cells transfected with wild-type or mutant FUS using 32p labeled oligo-nucleotides as shown in Figure 6B as probe. The arrow points to protein-DNA complexes complexes (A). For ChIP assay, using FUS or Flag antibody or IgG as control and interacting DNAs were immunoprecipitated and amplified by PCR using primers targeted to the promoter region (−2931 to −2712) of MnSOD gene. Total genomic DNA obtained after transfection was parallel-amplified by using the same primer as input control (B). The specificity of FUS binding to the MnSOD promoter was verified by amplifying the same DNAs with the primer set selected from the untargeted region of MnSOD promoter (−1823 to −1613) (B). Coimmunoprecipitation experiment was performed using nuclear extract of cells transfected with wild-type or mutant FUS expression vector using Flag antibody (C). Flag, Sp1, and NPM was detected by Western blotting analysis using antibodies specific for these proteins and IgG was used as negative control. Equal sample loading was confirmed by probing the same membrane with anti-IgG (C, top panel). FUS interacting proteins were also affinity purified from the nuclear extracts of cells transfected with wild-type or mutant-type FUS expression vector or empty vector using anti-Flag affinity column. Flag binding proteins were eluted with 0.5 M glycine solution, analyzed by SDS-PAGE, and detected by Western blotting (C, bottom panel). Coimmunoprecipitation was performed with cell lysates incubated in the absence (D, top panel) or presence (D, bottom panel) of DNase for 1 h at 37°C.