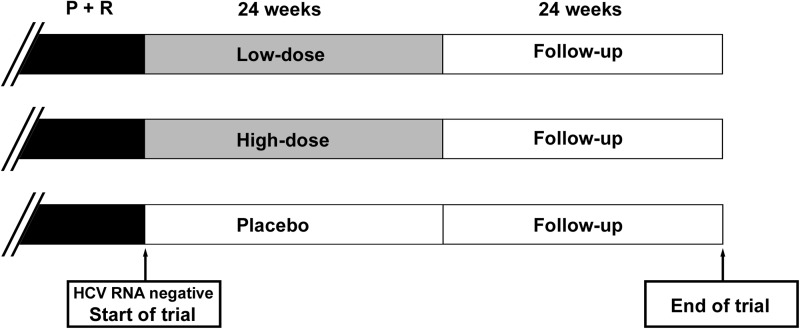
FIG. 1.
Clinical trial design. Hepatitis C patients infected with genotype 1b and received pegylated interferon plus ribavirin (P+R) treatment were included, provided that virological clearance was achieved at the end of therapy. Oral interferon was given for 24 weeks (low dose 500 IU/day, high dose 1,500 IU/day, or placebo) followed by 24 weeks of follow-up.