ABSTRACT
We analyzed almost complete 18S rDNA sequences of 10 bovine Eimeria species, namely Eimeria alabamensis, E. auburnensis, E. bovis, E. bukidnonensis, E. canadensis, E. cylindrica, E. ellipsoidalis, E. subspherica, E. wyomingensis and E. zuernii. Although these sequences showed intraspecific variation in 8 species, the sequences of each species were clustered in monophyletic groups in all species, except E. auburnensis. The sequences constituted 3 distinct clusters in a phylogenetic tree with relatively high bootstrap values; however, the members including each cluster shared no similarities in oocyst morphology.
Keywords: 18S rDNA, cattle, Eimeria, oocysts, sequences
Bovine coccidiosis causes acute and chronic intestinal damage to the cattle and causes considerable economic losses to the cattle industry. More than 20 Eimeria species have been identified, and least 13 Eimeria species are confirmed as causative agents of this disease [9]. Pathogenicity to the host differs among the different Eimeria spp., and the 2 species, E. bovis and E. zuernii, are highly pathogenic and sometimes cause severe diarrhea in calves. Identification of Eimeria species has been largely based on the morphology of sporulated oocysts; however, only some species show morphological resemblance with each other. Therefore, molecular characterization is essential to accurately delimit species and infer phylogenic relationships among species. The small subunit ribosomal RNA (SSU rRNA) gene is a well-known marker for molecular characterization. Several studies have addressed the phylogenetic relationships based on 18S rDNA sequences and the morphology of Eimeria species, which parasitize domestic fowl [2], rodents [16] and rabbits [6]. However, the complete 18S rDNA sequences of only 2 bovine Eimeria species (E. bovis and E. alabamensis) have been analyzed and, to date, no study has reported the relationships between phylogeny of bovine Eimeria spp. on the basis of 18S rDNA sequences and similarities in their oocyst morphology. The purpose of this study was to analyze almost the complete 18S rDNA sequence of 10 bovine Eimeria species and determine the phylogenetic relationships among these species.
MATERIALS AND METHODS
Eimeria oocysts: One hundred sixty eight fecal samples were obtained from cattle in Iwate, Aichi, Hyogo and Nagasaki prefectures in Japan. Samples were evaluated for Eimeria oocysts using the sucrose centrifugal floatation technique. Eimeria oocysts were isolated from positive samples, sporulated in 2% potassium dichromate solution at room temperature and morphologically identified according to the procedure described by Levine and Ivens [10, 11]. Oocysts of 10 Eimeria species, namely E. alabamensis, E. auburnensis, E. bovis, E. bukidnonensis, E. canadensis, E. cylindrica, E. ellipsoidalis, E. subspherica, E. wyomingensis and E. zuernii, were detected, and a total of 119 oocysts were used in this study (Table 1).
Table 1. Profile of Eimeria oocysts used in this study.
| Species | Oocyst | Location | |
|---|---|---|---|
| Isolate code | Numbers | ||
| E. alabamensis | Eal-isolate1a)-1~10 b) 10 | 10 | Hyogo |
| E. aubrnensis | Eau-isolate1-1~7 | 7 | Iwate |
| Eau-isolate2-1~2 | 2 | Aichi | |
| Eau-isolate3-1~4 | 4 | Nagasaki | |
| Eau-isolate4-1~2 | 2 | Hyogo | |
| E. bovis | Ebo-isolate1-1~4 | 4 | Iwate |
| Ebo-isolate2-1~2 | 2 | Aichi | |
| Ebo-isolate3-1~2 | 2 | Aichi | |
| Ebo-isolate4-1~3 | 3 | Aichi | |
| Ebo-isolate5-1 | 1 | Aichi | |
| Ebo-isolate6-1~4 | 4 | Nagasaki | |
| Ebo-isolate7-1~2 | 2 | Hyogo | |
| E. bukidnonensis | Ebu-isolate1-1~4 | 4 | Iwate |
| Ebu-isolate2-1~3 | 3 | Nagasaki | |
| Ebu-isolate3-1~2 | 2 | Hyogo | |
| Ebu-isolate4-1~3 | 3 | Hyogo | |
| E. canadendsis | Eca-isolate1-1~10 | 10 | Iwate |
| E. cylindrica | Ecy-isolate1-1~4 | 4 | Iwate |
| Ecy-isolate2-1~7 | 7 | Nagasaki | |
| Ecy-isolate3-1~2 | 2 | Nagasaki | |
| E. ellipsoidaris | Ee-isolate1-1~2 | 2 | Iwate |
| Ee-isolate2-1~2 | 2 | Iwate | |
| Ee-isolate3-1 | 1 | Nagasaki | |
| Ee-isolate4-1~5 | 5 | Hyogo | |
| E. subspherica | Es-isolate1-1~3 | 3 | Nagasaki |
| Es-isolate2-1~7 | 7 | Nagasaki | |
| E. wyomingensis | Ew-isolate1-1~10 | 10 | Hyogo |
| E. zuernii | Ez-isolate1-1~4 | 4 | Iwate |
| Ez-isolate2-1~2 | 2 | Aichi | |
| Ez-isolate3-1~4 | 4 | Aichi | |
| Ez-isolate4-1 | 1 | Nagasaki | |
| Total no. | 119 | ||
a) “isolate” means a fecal sample. b) “1~nos.” mean oocyst no.
DNA extraction: Total DNA was extracted from each oocyst. Briefly, a single oocyst was placed into a PCR tube using a capillary micropipette and an inverted microscope, washed 5 times with resuspension in 100 µl of 0.05% Tween 20 solution and centrifugation at 10,000 rpm for 1 min and treated with 100 µl of 0.5% sodium hypochlorite for 1 hr at 4°C. After washing, the oocyst was treated with 100 µl saturated sodium chloride for 1 hr at 55°C [5]. After washing several times with 100 µl MilliQ water and centrifugation at 10,000 rpm for 1 min, total DNA was extracted by exposing the oocyst to 5 cycles of freezing for 3 min at −80°C and thawing for 1 min at 60°C. The volume of total DNA solution was adjusted to 6.65 µl with MilliQ water.
PCR, sequencing and phylogenic analysis: Two primer sets for nested PCR, TK2 and ets2 for the first PCR, and TK1 and 18S-14 for the second PCR, were designed to amplify an approximately 1,800-bp fragment of 18S rDNA on the basis of the reference sequences of E. bovis (EBU77084), E. alabamensis (AF291427), E. gruis (AB544361) and E. reichenowi (AB544355) (Table 2). The first PCR reaction was performed in a 25-µl reaction volume containing 6.65 µl of DNA template, 0.2 mM of each dNTP, 0.1 mM of each primer, 0.25 U of KOD FX neo DNA polymerase (Toyobo, Osaka, Japan) and the manufacturer-supplied reaction buffer. The thermal program was 94°C for 10 min, 20 cycles of 98°C for 10 sec, 67°C for 3 min and 72°C for 10 min. The second PCR reaction was performed using the same reaction mix as the first PCR, except 1 µl of the first PCR reactions was used as template DNA with Go Taq DNA polymerase (Promega, Madison, WI, U.S.A.). The thermal program was 94°C for 2 min, 30 cycles of 98°C for 10 sec, 56°C for 10 sec and 72°C for 90 sec, and 72°C for 7 min. PCR amplicons were purified using the High Pure PCR Cleanup Micro Kit (Roche Diagnostic, Mannheim, Germany) and directly sequenced in both directions using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, U.S.A.) using the additional forward primers 18S-9 and 18S-13 and the reverse primers Eb1-R and 18S-12 (Table 2). The resultant sequencing ladders were read using the ABI PRISM 3500-Avant Genetic Analyzer (Applied Biosystems). 18S rDNA sequences were aligned using Clustal X version 2.0 software [7]. A neighbor–joining (NJ) phylogram was constructed using PAUP 4.0b10 [14]. For phylogenetic tree construction, the sequence of Eimeria tenella(accession no. AF026388) was used as an outgroup. Bootstrap analyses were performed using 1,000 replicates.
Table 2. Primers used in this study.
| Primers | Sequences |
|---|---|
| TK2 | 5′-GGT TGA TCC TGC CAG TAG TC-3′ |
| ets2 | 5′-AAT CCC AAT GAA CGC GAC TCA-3′ |
| TK1 | 5′-AGT AGT CAT ATG CTT GTC TC-3′ |
| 18S-14 | 5′-ACG GAA ACC GTG TTA CGA CT-3′ |
| 18S-9 | 5′-ACA ATT GGA GGG CAA GTC T-3′ |
| 18S-13 | 5′-ATG CCT ACC TTG GCT TCT CC-3′ |
| Eb1-R | 5′-AGC CTG CTT GAA ACA CTC T-3′ |
| 18S-12 | 5′-GAA CCG TAG TTC CCG ATC CT-3′ |
RESULTS
The 18S rDNA sequences of 10 bovine Eimeria species were determined and deposited into the DNA Data Bank of Japan (DDBJ) with accession nos. AB769547–AB769665. The sequences ranged from 1,761 bp for E. ellipsoidalis to 1,774 bp for E. subspherica and showed intraspecific variation in 8 species, except for E. wyomingensis and E. zuernii (Table 3). The variants included 6 for 10 oocysts of E. alabamensis, 3 for 15 oocysts of E. auburnensis, 2 for 18 oocysts of E. bovis, 4 for 12 oocysts of E. bukidnonensis, 3 for 10 oocysts of E. canadensis, 5 for 13 oocysts of E. cylindrical, 3 for 10 oocysts of E. ellipsoidalis and 6 for 10 oocysts of E. subspherica. Sequence variation was relatively low (<0.2%) in 6 species, except for E. alabamensis (<1.2%) and E. bukidnonensis (<0.5%). The oocysts of E. wyomingensis and E. zuernii, which showed no intraspecific variation in their 18S sequences, were obtained from 1 and 4 fecal samples (isolates), respectively; moreover, the feces from which E. zuernii oocysts were isolated were derived from 3 distinct locations (Iwate, Aichi and Nagasaki) (Table 1). Oocysts of E. alabamensis and E. canadensis, which showed intraspecific variation in their sequences, were each obtained from single fecal samples. The 18S rDNA sequences showed pairwise similarities in the range of 99.5–99.9% (E. auburnensis vs. E. cylindrica) and 96.7–97.0% (E. alabamensis vs. E. ellipsoidalis) (Table 3).
Table 3. Pairwise similarity (%) of 18S rDNA sequences among 10 bovine Eimeria species.
| Species | (bp) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. E. alabamensis | 1765–1769 | 98.8–100 | |||||||||
| 2. E. aubrnensis | 1764 | 97.0–97.4 | 99.8–100 | ||||||||
| 3. E. bovis | 1763–1764 | 96.7–97.2 | 98.8–99.1 | 99.8–100 | |||||||
| 4. E. bukidnonensis | 1768–1769 | 98.4–99.0 | 97.3–97.6 | 96.9–97.3 | 99.5–100 | ||||||
| 5. E. canadendsis | 1763–1764 | 96.8–96.9 | 98.9–99.0 | 98.2–98.5 | 96.8–97.0 | 99.9–100 | |||||
| 6. E. cylindrica | 1763–1764 | 97.0–97.4 | 99.5–99.9 | 98.7–99.1 | 97.3–97.5 | 98.8–98.9 | 99.8–100 | ||||
| 7. E. ellipsoidaris | 1761–1763 | 96.7–97.0 | 98.8–98.9 | 99.3–99.7 | 96.9–97.2 | 98.3–98.4 | 98.8–98.9 | 99.8–100 | |||
| 8. E. subspherica | 1773–1774 | 98.4–98.9 | 97.3–97.6 | 96.8–97.2 | 98.3–98.6 | 97.0–97.3 | 97.1–97.3 | 96.9–97.1 | 99.8–100 | ||
| 9. E. wyomingensis | 1764 | 96.8–97.2 | 99.5–99.7 | 98.8–99.0 | 97.0–97.2 | 98.8 | 99.5–99.7 | 98.7–98.8 | 97.1–97.2 | 100 | |
| 10. E. zuernii | 1763 | 96.9–97.2 | 98.9–99.0 | 99.4–99.7 | 97.1–97.3 | 98.4 | 98.8–99.0 | 99.4–99.5 | 97.1–97.2 | 98.9 | 100 |
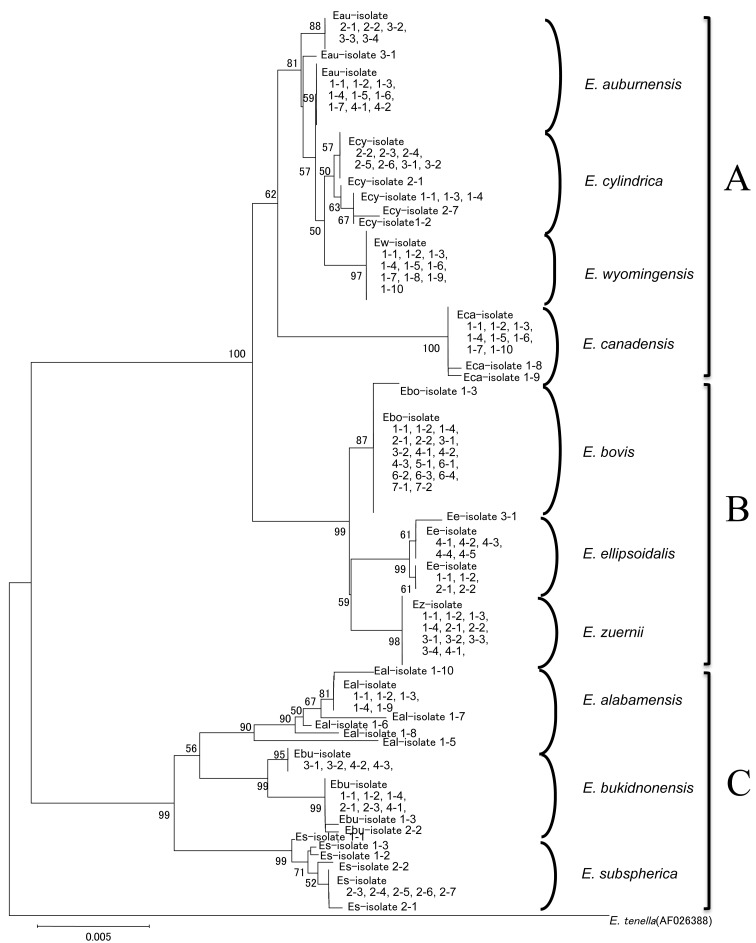
In the phylogenetic tree, the sequences of 9 Eimeria species, except for that of E. auburnensis, were clustered in monophyletic groups (clades) formed for each species, and the clades of E. alabamensis and E. bukidnonensis consisted of phylogenetically distant variants (Fig. 1). Ten Eimeria species constituted 3 distinct clusters with relatively high bootstrap values: cluster A contained of E. auburnensis, E. canadensis, E. cylindrica and E. wyomingensis, cluster B contained of E. bovis, E. ellipsoidalis and E. zuernii, and cluster C contained of E. alabamensis, E. bukidnonensis and E. subspherica.
Fig. 1.
Neighbor–joining phylogenic tree based on 18S rDNA sequences of 10 bovine Eimeria species. Each isolate shows a sequence obtained from total DNA extracted from a single oocyst profiled in Table 1. The numbers in the branches represent bootstrap values of more than 50%. A, B and C represent the monophyletic groups, including the sequences of Eimeria spp.
DISCUSSION
Few studies have examined the relationships between the phylogeny of Eimeria species based on 18S rDNA sequences and their biological traits, such as oocyst morphology. Phylogenetic relationships among Eimeria spp., which parasitize domestic fowl, correlate with similarities based on their oocyst size and shape [2]. Rabbit Eimeria species formed a phylogenic tree that was closely linked to the presence/absence of oocyst residuum and showed no relevant similarities to other morphological characteristics, such as oocyst size and shape [6]. Although more than 13 Eimeria species parasitizing cattle possess biological characteristics unique to each species, few studies have addressed the relationships between their biological traits and molecular phylogenies. In this study, we clarified the 18S rDNA sequences of 10 Eimeria spp. detected from cattle in Japan and found that these species were clearly divided into 3 monophyletic groups, although the members clustered in each group shared no similarities in oocyst morphology. It is reported that 6 bovine Eimeria species are clustered in 3 distinct groups in a phylogenetic analysis based on nuclear ribosomal ITS1 sequences, although E. ellipsoidalis belonged to a group different from that clustered in this study [4]. Interestingly, one of the groups (cluster B in this study) included the highly pathogenic E. bovis and E. zuernii. Similarly, these 2 species also constituted the same monophyletic group in analysis based on ITS1 sequences [4]. Furthermore, the 18S rDNA sequences indicated that the highly pathogenic E. tenella and E. necatrix in domestic fowl also belonged to identical phylogenetic groups [2]. The 2 chicken Eimeria species shared common characteristics, and their second-generation meronts develop within the lamina propria of the lower intestinal tracts [9]. The biological traits common to both E. bovis and E. zuernii are to parasitize the regions of the lower intestinal tracts, such as the cecum and colon and to produce first-generation meronts of large size [9]; these traits suggest that the 2 species may have evolved from a common ancestor, although E. ellipsoidalis which belongs to the same clade does not possess these traits.
The results of our study revealed intraspecific sequence diversity in most bovine Eimeria species, particularly crucial differences (98.8% and 99.5%) in E. alabamensis and E. bukidnonensis. Nuclear ribosomal DNA in eukaryotic genomes consists of multiple tandemly arranged repeats, and the copies are thought to undergo concerted evolution, because of unequal exchange and gene conversion within and between the same chromosome loci and appear to be continuously homogenized [1, 3]. However, several apicomplexan species including Eimeria species shared distinct types of 18S rDNA with sequence identities ranging from 89% for Plasmodium falciparum [12] to 99% for Cryptosporidium parvum [8], Babesia bigemina [13] and Eimeria mitis [15]. Although the distinct types are thought to be intragenomic variants, we were unable to clarify whether intraspecific sequence diversity coexists in a single genome since total DNA extracted from a single oocyst produced unique 18S rDNA sequences. Further molecular studies are required to examine this possibility.
ACKNOWLEDGMENTS
We are extremely grateful to Dr. Hiromitsu Ooba of NOSAI Aichi, Dr. Naoshi Yamamoto of NOSAI Hyogo, and Dr. Taisuke Tominaga of NOSAI Nagasaki for providing bovine fecal samples.
References
- 1.Arnheim N. 1983. Concerted evolution of multigene families. pp. 38–61. In: Evolution of Genes and Proteins (Nei, M. and Koehn, R. K. eds.), Sinauer, Sunderland. [Google Scholar]
- 2.Barta J. R., Martin D. S., Liberator P. A., Dashkevicz M., Anderson J. W., Feighner S. D., Elbrecht A., Perkins-Barrow A., Jenkins M. C., Danforth H. D., Ruff M. D., Profous-Juchelka H. 1997. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 83: 262–271. doi: 10.2307/3284453 [DOI] [PubMed] [Google Scholar]
- 3.Hillis D. M., Dixon M. T. 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Q. Rev. Biol. 66: 411–453. doi: 10.1086/417338 [DOI] [PubMed] [Google Scholar]
- 4.Kawahara F., Zhang G., Mingala C. N., Tamura Y., Koiwa M., Onuma M., Nunoya T. 2010. Genetic analysis and development of species-specific PCR assays based on ITS-1 region of rRNA in bovine Eimeria parasites. Vet. Parasitol. 174: 49–57. doi: 10.1016/j.vetpar.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Kaya G., Dale C., Maudlin I., Morgan K. 2007. A novel procedure for total nucleic acid extraction from small numbers of Eimeria species oocysts. Turkiye Parazitol. Derg. 31: 180–183 [PubMed] [Google Scholar]
- 6.Kvicerová J., Pakandl M., Hypsa V. 2008. Phylogenetic relationships among Eimeria spp. (Apicomplexa, Eimeriidae) infecting rabbits: evolutionary significance of biological and morphological features. Parasitology 135: 443–452. doi: 10.1017/S0031182007004106 [DOI] [PubMed] [Google Scholar]
- 7.Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. doi: 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 8.Le Blancq S. M., Khramtsov N. V., Zamani F., Upton S. J., Wu T. W. 1997. Ribosomal RNA gene organization in Cryptosporidium parvum. Mol. Biochem. Parasitol. 90: 463–478. doi: 10.1016/S0166-6851(97)00181-3 [DOI] [PubMed] [Google Scholar]
- 9.Levine N. D. 1985. Apicomplexa: The coccidia proper. pp.130–232. In: Veterinary Protozoology. The Iowa State University Press, Ames. [Google Scholar]
- 10.Levine N. D., Ivens V. 1967. The sporulated oocysts of Eimeria illinoisensis n. sp. and of other species of Eimeria of the ox. J. Protozool. 14: 351–360 [DOI] [PubMed] [Google Scholar]
- 11.Levine N. D., Ivens V. 1970. The Coccidian Parasites (Protozoa, Sporozoa) of Ruminants. Illinois Biology Monograph No. 44. University of Illinois Press, London. [Google Scholar]
- 12.McCutchan T. F., de la Cruz V. F., Lal A. A., Gunderson J. H., Elwood H. J., Sogin M. L. 1988. Primary sequences of two small subunit ribosomal RNA genes from Plasmodium falciparum. Mol. Biochem. Parasitol. 28: 63–68. doi: 10.1016/0166-6851(88)90181-8 [DOI] [PubMed] [Google Scholar]
- 13.Reddy G. R., Chakrabarti D., Yowell C. A., Dame J. B. 1991. Sequence microheterogeneity of the three small subunit ribosomal RNA genes of Babesia bigemina: expression in erythrocyte culture. Nucleic Acids Res. 19: 3641–3645. doi: 10.1093/nar/19.13.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swofford D. L. 2001. PAUP*. Phylogenetic analysis using parasimony and other methods ver. 4.0beta. Sunderland, Massachussetts: Sinauer Associates. [Google Scholar]
- 15.Vrba V., Poplstein M., Pakandl M. 2011. The discovery of the two types of small subunit ribosomal RNA gene in Eimeria mitis contests the existence of E. mivati as an independent species. Vet. Parasitol. 183: 47–53. doi: 10.1016/j.vetpar.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Duszynski D. W. 2001. Phylogenetic relationships among rodent Eimeria species determined by plastid ORF470 and nuclear 18S rDNA sequences. Int. J. Parasitol. 31: 715–719. doi: 10.1016/S0020-7519(01)00136-9 [DOI] [PubMed] [Google Scholar]