ABSTRACT
Free fatty acids play an important role in regulating animal insulin secretion response. Acute elevated free fatty acids increased animal insulin secretion and glucose-stimulated insulin secretion. In the present study, we perfused the rat pancreas to explore the effect of unsaturated fatty acids on insulin secretion. The results showed that linoleic acid, γ-linolenic acid and arachidonic acid significantly stimulated insulin secretion. Glucose (10 mM) alone induced a biphasic insulin secretion response. The peak effluent insulin concentrations increased by 444% and 800% compared with the baseline in the first and second insulin secretion phases, respectively. Based on comparison of the percentage increases, arachidonic acid, γ-linolenic acid or linoleic acid increased glucose-induced insulin release by 555% and 934%, 522% and 995% and 463% and 1,105% in the first and second insulin secretion phases, respectively. However, the percentage increases of insulin secretion decreased significantly to 402% and 564% in the first and second phases in the rats fed a high-fat diet for 13 weeks. Linoleic acid alone stimulated a 391% increase in the peak insulin concentration compared with the baseline in the rats fed a normal diet. The peak insulin concentration decreased significantly to183% in the rats fed a long-term high-fat diet. All the results suggested that unsaturated fatty acids stimulated insulin secretion and additively increased glucose-induced insulin secretion in the perfused rat pancreas. However, the rats fed a high-fat diet had a decreased linoleic acid-induced insulin secretion response.
Keywords: arachidonic acid, insulin, insulin secretion, linoleic acid, pancreas
Obesity is associated with a variety of conditions, including glucose intolerance, diabetes mellitus, hypertension, dystocia, osteoarthritis and respiratory distress [22]. In our previous report, the new thiazolidinedione (TZD) antihyperglycemic agonist rosiglitazone increased animal insulin secretion response via the phosphatidylinositol 3-kinase pathway in the perfused rat pancreas [21]. In addition, it was reported that TZDs produced a potent insulin-sensitizing activity in vivo and reduced blood glucose, insulin and triglyceride levels in insulin resistant animal models and in type 2 diabetic patients [6, 13, 16]. TZDs were high-affinity ligands for the peroxisome proliferator-activated receptor γ (PPARγ). The relative potency of TZDs to bind and to activate PPARγ in vitro correlated with their antidiabetic action in vivo [2]. It was found that polyunsaturated fatty acids were ligands for PPARα and δ [8]. The natural ligands for PPARγ are linoleic acid, γ-linolenic acid, arachidonic acid and eicosapentaenoic acid (EPA) [20]. Linoleic acid is a polyunsaturated essential fatty acid called omega-6 fatty acid. In physiological literature, it has been used in the biosynthesis of prostaglandins and reported to be rich in the lipids of cell membranes. There is evidence that free fatty acids play an important role in regulating animal insulin secretion response and glucose homeostasis. It was recognized in a previous study that elevated plasma free fatty acids had both stimulatory and inhibitory effects on insulin secretion [8]. Elevated free fatty acids were proved to enhance glucose-stimulated insulin secretion in fasted rats. Prolonged exposure to elevated fatty acids induced an impairment of animal insulin secretion in β-cells secretion function, whereas an acute exposure was found to enhance insulin secretion [8].
In addition, saturated fatty acids induced lipoapoptosis in human β-cells, whereas unsaturated fatty acids had no effect [8]. The insulinotropic effect of free fatty acids was profoundly influenced by the chain length and degree of saturation of individual fatty acids under certain circumstances [18]. Long-chain and saturated fatty acids were more effective than medium-chain and unsaturated fatty acids [9, 15, 19]. Therefore, the aim of the present study was to explore the effect of unsaturated fatty acids on animal insulin secretion. For this purpose, the rat pancreas was perfused with linoleic acid at different dosage levels or linoleic acid with 10 mM glucose to determine the effect of polyunsaturated free fatty acid on the function of animal β-cells.
MATERIALS AND METHODS
Animals and chemicals: Male Sprague-Dawley rats, which originated from the Animal Center of the National Science Council of the R.O.C., weighing 250–350 g were used in the experiments. The rats were kept at room temperature (about 25°C) in plastic cages under a cycle of 12 hr light. All the rats were given free access to tap water and fed ad libitum with commercial chow (Fwusow, Sha-Lu, Taichung, R.O.C.) or were fed with a high-fat diet 30% (Purified TestDiet: Fat (lipid) Series (using Basal Diet 5755), PMI, Richmond, IN, U.S.A.). Rats were fasted overnight (over 12 hr) prior to the experiments and anesthetized with intraperitoneal injection of pentobarbital sodium (65 mg/kg; MTC Pharmaceuticals, Cambridge, ON, Canada). Access to the pancreata was gained through a ventral midline incision, and the celiac arteries and portal veins were cannulated with 0.625 mm and 1.2 mm (internal diameter), polyvinyl tubing, respectively. The rats were maintained at 37°C throughout the experiments. Krebs-Ringer bicarbonate buffer (KRB), supplemented with 5.5 mM glucose, 10 mM N-2-hydroxyethyl-piperazine-N’-2-ethanesulfonic acid (HEPES), 0.1% dextran and 0.2% bovine serum albumin, was used as the perfusate (basal medium). KRB solution was continuously aerated with 95% O2–5% CO2, and the pH value was maintained at 7.4. All the chemicals were purchased from Sigma Chemical (St. Louis, MO, U.S.A.), except for linoleic acid, γ-linoleic acid and arachidonic acid, which were purchased from Nu-Chek Prep, Inc. (Elysian, MN, U.S.A.). The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of National Chung Hsing University.
Pancreatic perfusion: In situ live rat pancreatic perfusion was performed at 37°C by using a method of Grodsky and Fanska [10]. The pancreatic perfusion was maintained at a flow rate of 1 ml/min. After cannulation of the celiac artery and portal vein and performing the necessary preparations for rat pancreatic perfusion, the first 20 min of perfusion were considered an equilibration period. Subsequently, the effluent fluid was collected every min from the cannula of the portal vein for 50 or 55 min. In experiment 1, after a baseline period of 10 min, the perfusate containing 100 µM of linoleic acid, γ-linoleic acid or arachidonic acid was administered for 30 min followed by administration of the basal medium for 10 min, respectively. In experiment 2, after a baseline period of 10 min, the perfusate containing linoleic acid (10, 50, 100 or 150 µM) was administered for 30 min followed by administration of the basal medium for 10 min. In experiment 3, the perfusate containing 10 mM glucose with or without linoleic acid (100 µM) was administered for 30 min, and this was followed by a basal medium washout for the last 10 min. In experiment 4, the perfusate containing 10 mM glucose with 100 µM of linoleic acid, γ-linolenic acid or linoleic acid was administered for 30 min, and this was followed by a basal medium washout for the last 10 min. In experiment 5, the perfusate containing 100 µM linoleic acid with or without 10 mM glucose was administered for 30 min into the rats fed a long-term high-fat diet for 13 weeks [1], and this was followed by a basal medium washout for the last 10 min. The collected effluent fluid was kept at 4°C and subsequently assayed within 12 hr for insulin by using radioimmunoassay (RIA) as previously described by Hale and Randle (1963). Rat insulin was used as the standard for the RIA.
Data expression and statistical analysis: Data for effluent insulin concentrations were expressed as mean percentages of the baseline level (mean of 12 baseline values) ± SE. Data were analyzed by using analysis of variance (ANOVA) to determine the significance of treatment and time. The interaction between treatment and time was used as an error term to determine the effect of treatment. The significance of treatment was determined from the conservative F value. Tukey’s highly significant different test was used to determine the differences between treatments for which the ANOVA indicated a significant (P<0.05) F ratio. For analyzing the first phase (from 2–7 min) and second phase (8–30 min) insulin secretions during glucose perfusion, the areas under curves (AUC) of the percentage increases over baseline were calculated and compared with the Student’s tests. A P of<0.05 was considered statistically significant.
RESULTS
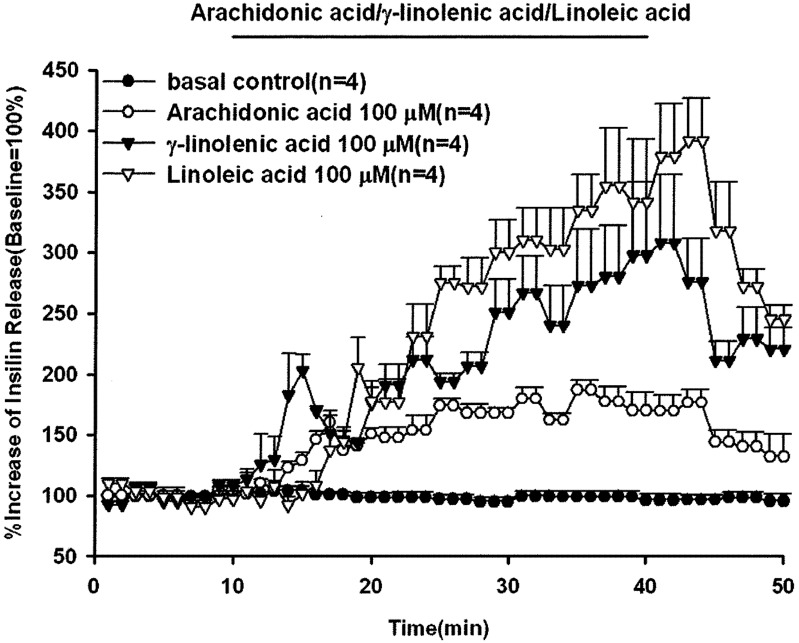
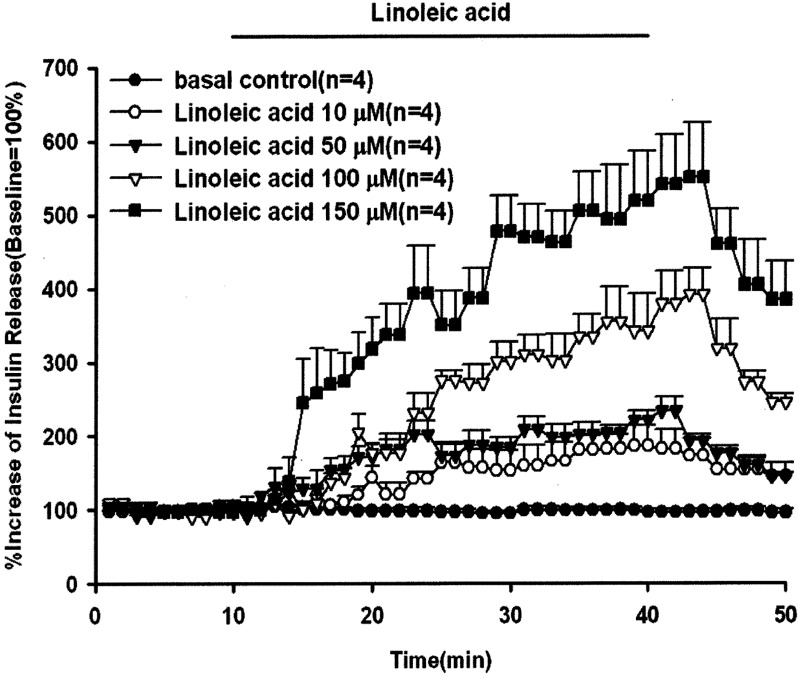
In order to explore the stimulatory effect of unsaturated fatty acids on animal insulin secretion, the rat pancreas was perfused with 100 µM of linoleic acid, γ-linolenic acid or arachidonic acid for 30 min. The results in Fig.1 show that all the perfused unsaturated fatty acids significantly stimulated animal insulin secretion with a gradually increasing pattern of release. The peak effluent insulin concentrations increased by 391%, 307% and 186% compared with the baseline for linoleic acid, γ-linolenic acid and arachidonic acid, respectively. The basal insulin concentrations were 4,172 ± 277, 2,384 ± 802, 3,541 ± 508 and 1,199 ± 127 pg/ml for the control, arachidonic acid, γ-linolenic acid and linoleic acid groups, respectively. The dose-dependent effects of linoleic acid on insulin secretion are shown in Fig. 2. The peak effluent insulin concentrations were increased by 186%, 240%, 391% and 540% compared with the basal control group for 10, 50, 100 and 150 µM of linoleic acid, respectively. Linoleic acid (10, 50, 100 and 150 µM) significantly stimulated insulin secretion in a dose-dependent manner. The insulin secretion response declined after discontinuation of linoleic acid perfusion. The basal effluent insulin concentrations were 4,172 ± 277, 1,660 ± 352, 1,910 ± 172, 1,199 ± 127 and 4,271 ± 1,739 pg/ml for the control group and 10, 50, 100 and 150 µM of linoleic acid groups, respectively.
Fig. 1.
Effects of arachidonic acid, γ-linolenic acid and linoleic acid on insulin secretion. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, arachidonic acid, or γ-linolenic acid or linoleic acid (100 µM) was administered for 30 min followed by basal medium (KRB) perfusion. The horizontal line indicates the presence of arachidonic acid, γ-linolenic acid and linoleic acid. Values are means ± SE (n=4). Baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 2,384 ± 802 pg/ml, 3,541 ± 508 pg/ml and 1,199 ± 127 pg/ml for the control, arachidonic acid, γ-linolenic acid and linoleic acid groups, respectively.
Fig. 2.
Effect of linoleic acid on insulin release. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, linoleic acid (10, 50, 100 and 150 µM) was administered for 30 min followed by basal medium (KRB) perfusion. The horizontal line indicates the presence of linoleic acid. Values are means ± SE (n=4). Baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 1,660 ± 352 pg/ml, 1,910 ± 172 pg/ml, 1,199 ± 127 pg/ml and 4,271 ± 1,739 pg/ml for the control group and 10, 50, 100 and 150 µM linoleic acid groups, respectively.
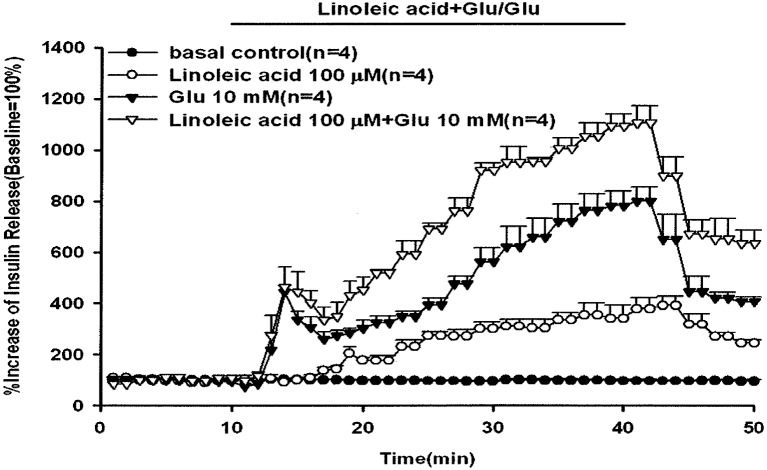
As shown in Fig. 3, glucose (10 mM) with or without linoleic acid (100 µM) was infused via in situ pancreatic perfusion. Glucose (10 mM) alone induced a biphasic insulin secretion response. The peak effluent insulin concentrations increased by 444% and 800% compared with the baseline in the first (11–20 min) and second (21–50 min) insulin secretion phases, respectively. After the administration of linoleic acid in perfusate containing 10 mM glucose, the glucose-induced second phase of insulin secretion was additively increased compared with glucose alone (P<0.01). Comparison of the peak effluent insulin concentration with administration of linoleic acid (100 µm) with the baseline showed 463% and 1,105% increases in insulin secretion in the first and second phases of insulin secretion, respectively. The baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 1,199 ± 127 pg/ml, 2,881 ± 775 pg/ml and 1,626 ± 298 pg/ml for the control, 100 µM linoleic acid, 10 mM glucose and 100 µM linoleic acid with 10 mM glucose groups, respectively.
Fig. 3.
Effect of linoleic acid on glucose-induced insulin release. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 10 mM glucose with or without 100 µM linoleic acid was administered for 30 min followed by basal medium (KRB) perfusion. The horizontal line indicates the presence of and linoleic acid and glucose. Values are means ± SE (n=4). Baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 1,199 ± 127 pg/ml, 2,881 ± 775 pg/ml and 1,626 ± 298 pg/ml for the control, 100 µM linoleic acid, 10 mM glucose and 100 µM linoleic acid with 10 mM glucose groups, respectively.
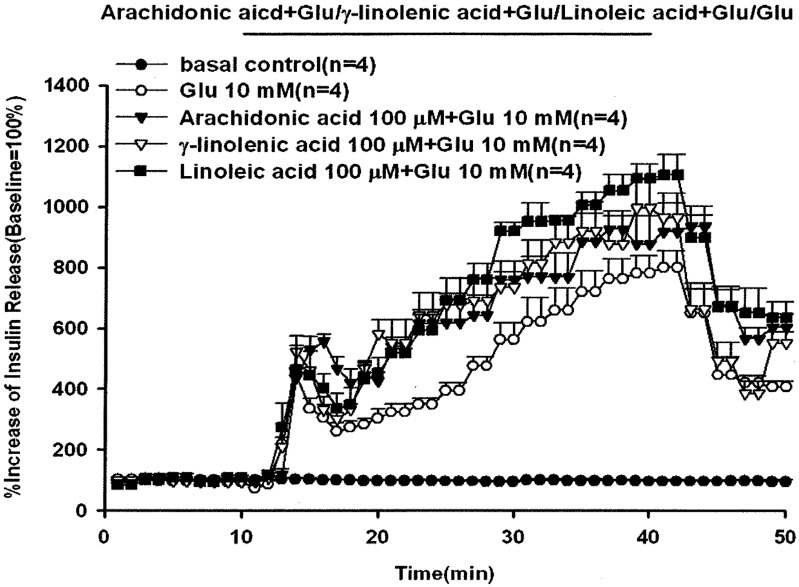
Comparison of the percentage increases in peak effluent concentrations in Fig. 4 showed that arachidonic acid, γ-linolenic acid and linoleic acid increased glucose-induced insulin release by 555% and 934%, 522% and 995% and 463% and 1,105% compared with the basal control group in the first and second insulin secretion phases, respectively. The peak effluent insulin concentrations with 10 mM glucose alone increased by 444% and 800% compared with the baseline in the first and second insulin secretion phases. The baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 2,881 ± 775 pg/ml, 1,850 ± 189 pg/ml, 2,546 ± 198 pg/ml and 1,626 ± 298 pg/ml for the control, 10 mM glucose alone, 100 µM arachidonic acid with 10 mM glucose, 100 µM γ-linolenic acid with 10 mM glucose and 100 µM linoleic acid with 10 mM glucose groups, respectively.
Fig. 4.
Effects of arachidonic acid, γ-linolenic acid or linoleic acid on glucose-induced insulin release. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 10 mM glucose with arachidonic acid, γ-linolenic acid or linoleic acid (100 µM) was administered for 30 min followed by basal medium (KRB) perfusion. The horizontal line indicates the presence of arachidonic acid, γ-linolenic acid, linoleic acid and glucose, respectively. Values are means ± SE (n=4). Baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 2,881 ± 775 pg/ml, 1,850 ± 189 pg/ml, 2,546 ± 198 pg/ml and 1,626 ± 298 pg/ml for the control, 10 mM glucose, 100 µM arachidonic acid with 10 mM glucose, 100 µM γ-linolenic acid with 10 mM glucose and 100 µM linoleic acid with 10 mM glucose groups, respectively.
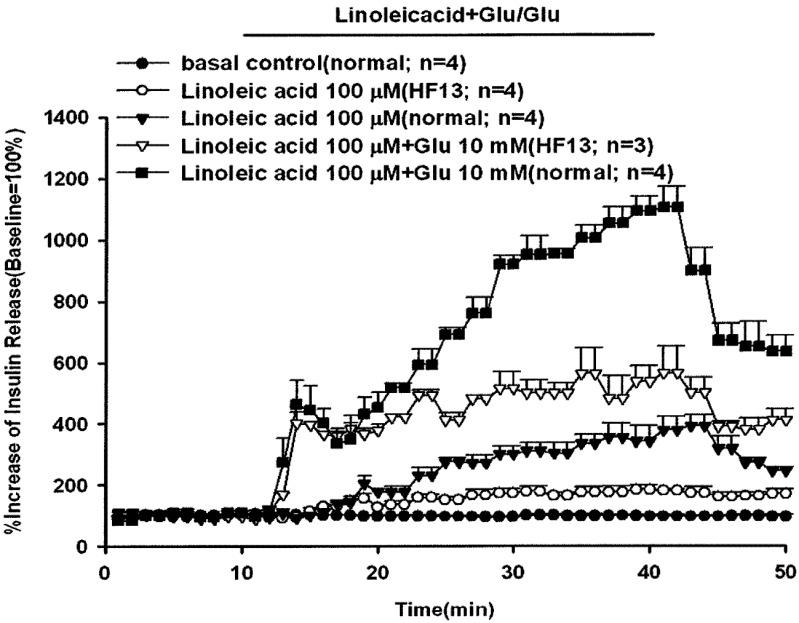
The effect of linoleic acid (100 µM) with or without glucose (10 mM) on insulin secretion in rats fed a long-term high-fat diet for 13 weeks is shown in Fig. 5. The peak effluent insulin concentrations increased by 463% (first phase) and 1,105% (second phase) compared with the baseline when linoleic acid was administered with 10 mM glucose in the rats fed a normal diet. After rats were fed a high-fat diet for 13 weeks (HF 13) and perfused with linoleic acid and glucose, the percentage increases in insulin secretion decreased significantly to 402% and 564% in the first and second phases, respectively. Linoleic acid alone stimulated a 391% increase in the peak insulin concentration compared with the baseline in the rats fed a normal diet. The percentage increase in peak effluent insulin concentration decreased significantly to 183% in the rats fed a high-fat diet. Rats fed a high-fat diet showed a significantly decreased stimulatory response of linoleic acid with or without glucose-induced insulin secretion. The baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 6,463 ± 2,373 pg/ml, 1,199 ± 127 pg/ml, 3,178 ± 318 pg/ml and 1,626 ± 298 pg/ml for the control, linoleic acid (high-fat diet), linoleic acid (normal diet), linoleic acid with glucose (high-fat diet) and linoleic acid with glucose (normal diet) groups, respectively.
Fig. 5.
Effect of linoleic acid with or without glucose on insulin release in rats fed a high-fat diet for 13 weeks (HF 13). In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 100 µM linoleic acid with or without 10 mM glucose was administered for 30 min followed by a basal medium (KRB) perfusion. The horizontal line indicates the presence of linoleic acid and glucose. Values are means ± SE (n=4). Baseline effluent concentrations of insulin were 4,172 ± 277 pg/ml, 6,463 ± 2,373 pg/ml, 1,199 ± 127 pg/ml, 3,178 ± 318 pg/ml and 1,626 ± 298 pg/ml for the control, linoleic acid (high-fat diet), linoleic acid (normal diet), linoleic acid with glucose (high-fat diet) and linoleic acid with glucose (normal diet) groups, respectively.
DISCUSSION
Obesity is a growing problem in companion animals as well as in humans. Overweight and obesity are linked to insulin sensitivity and subsequently in older pets to an increased risk of developing diabetes mellitus [11]. Studies have found that intake of polyunsaturated fatty acid is related to a lower risk of animal cardiovascular disease, hyperlipidemia, obesity and diabetes [15], whereas intake of saturated fatty acids and elevated free fatty acid concentrations are strongly linked to the development of animal obesity, insulin resistance and diabetes [4, 19]. In addition, increased intake of polyunsaturated fatty acids is associated with the improvement of animal insulin action and adiposity [19].
Fatty acids and lipid-derived substrates are natural ligands for PPAR. Furthermore, polyunsaturated fatty acids are ligands for PPARα and δ. Therefore, the natural PPARγ ligand linoleic acid was used to perfuse the rat pancreas in this study to explore the effect of polyunsaturated fatty acid on animal insulin secretion response.
There is evidence that free fatty acids play an important role in regulating insulin secretion response. Elevated plasma free fatty acids have both stimulatory and inhibitory effects on insulin secretion [7]. In addition, the plasma free fatty acid concentration closely influenced glucose-induced insulin secretion response in fasting rats [18]. Stein et al. also reported that infusion of an antilipolytic agent, nicotinic acid and lowering of the level of plasma free fatty acid decreased the glucose-induced insulin secretion response in fasting rats, but did not affect fed animals. Rats that were administered exogenous fatty acids and had elevated endogenous free fatty acid levels exhibited supranormal glucose-induced insulin secretion [18]. However, long-term exposure to elevated plasma free fatty acid induced human β−cell apoptosis (lipoapoptosis) and impaired glucose-stimulated insulin secretion response [14, 17]. Chronically elevated free fatty acid also inhibited animal insulin-stimulated peripheral glucose uptake and induced β−cell dysfunction in certain forms of type 2 diabetes [3].
Under normal physiological conditions, animal insulin secretion and biosynthesis are maintained at a stable balance in pancreatic β−cells. There is a rapid increase in gene translation and proinsulin biosynthesis that effectively replenishes the intracellular insulin stores after glucose stimulation [16]. However, long-term exposure to elevated free fatty acids significantly increased the basal insulin secretion rate and decreased intracellular insulin stores of β-cells, which may severely deplete the cytosolic insulin stores, since elevated free fatty acids inhibited glucose-induced proinsulin biosynthesis [5]. The results showed that rats fed a high-fat diet for 13 weeks had a higher baseline insulin concentration (6,463 ± 2,373 pg/ml) compared with the insulin concentration (1,199 ± 127 pg/ml) in rats fed a normal diet and lower glucose-induced insulin secretion (Fig. 5).
An acute exposure to elevated plasma free fatty acids was found to enhance insulin secretion within 48 hr [3, 9]. In addition, short-term elevated free fatty acids were proven to enhance glucose-stimulated insulin secretion in fasted rats [18]. The results showed that all the perfused unsaturated fatty acids stimulated animal insulin secretion with a gradually increasing pattern of release within the administration period (Fig. 1). The peak concentration of released insulin appeared at the end of fatty acid perfusion. Linoleic acid also increased glucose-stimulated insulin secretion in β cells (Fig. 3). This supported the idea that transiently elevated free fatty acid stimulated animal insulin secretion. The glucose-induced insulin secretion was significantly increased in the presence of linoleic acid. Thus, free fatty acid is an important stimulus-response coupling factor for triggering animal insulin secretion. However, chronic exposure to free fatty acid may induce a persistent insulin release and produce a hyperinsulinemic condition that may influence insulin sensitivity and β-cell function, as commonly found in obesity and type 2 diabetes [3]. After rats were fed a high-fat diet for 13 weeks (HF 13) and perfused with linoleic acid and glucose, the percentage increases in insulin secretion were significantly decreased in the first and second phases. The rats fed a high-fat diet showed a significantly decreased stimulatory response of linoleic acid with or without glucose-induced insulin secretion.
All the present results demonstrated that unsaturated fatty acids (linoleic acid, γ-linolenic acid and arachidonic acid) significantly stimulated insulin secretion and that linoleic acid stimulated insulin secretion in a dose-dependent manner. In addition, unsaturated fatty acids additively increased glucose-induced insulin secretion in the perfused rat pancreas. However, prolonged feeding of a high fat diet induced impairment of the linoleic acid stimulatory effect on insulin secretion.
REFERENCES
- 1.Baiges I., Palmfeldt J., Bladé C., Gregersen N., Arola L. 2010. Lipogenesis is decreased by grape seed proanthocyanidins according to liver proteomics of rats fed a high fat diet. Mol. Cell Proteomics 9: 1499–1513. doi: 10.1074/mcp.M000055-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger J., Bailey P., Biswas C., Cullinan C. A., Doebber T. W., Hayes N. S., Saperstein R., Smith R. G., Leibowitz M. D. 1996. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology 137: 4189–4195. doi: 10.1210/en.137.10.4189 [DOI] [PubMed] [Google Scholar]
- 3.Boden G., Chen X., Rosner J., Barton M. 1995. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44: 1239–1242. doi: 10.2337/diabetes.44.10.1239 [DOI] [PubMed] [Google Scholar]
- 4.Bollheimer L. C., Skelly R. H., Chester M. W., McGarry J. D., Rhodes C. J. 1998. Chronic exposure to free fattyacids reduces pancreatic β-cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J. Clin. Invest. 101: 1094–1101. doi: 10.1172/JCI420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden G. 1997. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10. doi: 10.2337/diabetes.46.1.3 [DOI] [PubMed] [Google Scholar]
- 6.Chaiken R. L., Eckert-Norton M., Pasmantier R., Boden G., Ryan I., Gelfand R. A., Lebovitz H. E. 1995. Metabolic effects of darglitazone, an insulin sensitizer, in NIDDM patients. Diabetologia 38: 1307–1312. doi: 10.1007/BF00401763 [DOI] [PubMed] [Google Scholar]
- 7.Eitel K., Staiger H., Brendel M. D., Brandhorst D., Bretzel R. G., Haring H. U., Kellerer M. 2002. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochem. Biophys. Res. Commun. 299: 853–856. doi: 10.1016/S0006-291X(02)02752-3 [DOI] [PubMed] [Google Scholar]
- 8.Forman B. M., Chen J., Evans R. M. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc. Natl. Acad. Sci. 94: 4312–4317. doi: 10.1073/pnas.94.9.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravena C., Mathias P. C., Ashcroft S. J. H. 2002. Acute effects of fatty acids on insulin secretion from rat and human islets of langerhans. J. Endocrinol. 173: 73–80. doi: 10.1677/joe.0.1730073 [DOI] [PubMed] [Google Scholar]
- 10.Grodsky G. M., Fanska R. E. 1975. The in vitro perfused pancreas. Methods Enzymol. 39: 364–372. doi: 10.1016/S0076-6879(75)39033-2 [DOI] [PubMed] [Google Scholar]
- 11.Häring T., Haase B., Zini E., Hartnack S., Uebelhart D., Gaudenz D., Wichert B. A. 2012. Overweight and impaired insulin sensitivity present in growing cats. J. Anim. Physiol. Anim. Nutr (Berl.) (in press). doi: 10.1111/j.1439-0396.2012.01322.x [DOI] [PubMed] [Google Scholar]
- 12.Itoh N., Okamoto H. 1980. Translational control of proinsulin synthesis by glucose. Nature 283: 100–102. doi: 10.1038/283100a0 [DOI] [PubMed] [Google Scholar]
- 13.Lebovitz H. E. 1993. Insulin mimetic and insulin-sensitizing drugs. Diabetes Res. Clin. Pract. 20: 89–91. doi: 10.1016/0168-8227(93)90001-L [DOI] [PubMed] [Google Scholar]
- 14.Lupi R., Dotta F., Marselli L., Del Guerra S., Masini M., Santangelo C., Patane G., Boggi U., Piro S., Anello M., Bergamini E., Mosca F., Di Mario U., Del Prato S., Marchetti P. 2002. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on uman pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51: 1437–1442. doi: 10.2337/diabetes.51.5.1437 [DOI] [PubMed] [Google Scholar]
- 15.Rivellese A. A., Lilli S. 2003. Quality of dietary fatty acids, insulin sensitivity and type 2 diabetes. Biomed. Pharmacother. 57: 84–87. doi: 10.1016/S0753-3322(03)00003-9 [DOI] [PubMed] [Google Scholar]
- 16.Saltiel A. R., Olefsky J. M. 1996. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 45: 1661–1669. doi: 10.2337/diabetes.45.12.1661 [DOI] [PubMed] [Google Scholar]
- 17.Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. 1998. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. 95: 2498–2502. doi: 10.1073/pnas.95.5.2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein D. T., Stevenson B. E., Chester M. W., Basit M., Daniels M. B., Turley S. D., McGarry J. D. 1997. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest. 100: 398–403. doi: 10.1172/JCI119546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storlien L. H., Higgins J. A., Thomas T. C., Brown M. A., Wang H. Q., Huang X. F., Else P. L. 2000. Diet composition and insulin action in animal models. Br. J. Nutr. 83:(Suppl. 1): S85–S90. doi: 10.1017/S0007114500001008 [DOI] [PubMed] [Google Scholar]
- 20.Xu H. E., Lambert M. H., Montana V. G., Parks D. J., Blanchard S. G. P., Brown J., Sternbach D. D., Lehmann J. M., Wisely G. B., Willson T. M., Kliewer S. A., Milburn M. V. 1999. Molecular recognition of fatty acids by peroxisome proliferator- γ Activated receptors. Mol. Cell. 3: 397–403. doi: 10.1016/S1097-2765(00)80467-0 [DOI] [PubMed] [Google Scholar]
- 21.Yang C., Chang T. J., Chang J. C., Liu M. W., Tai T. Y., Hsu W. H., Chuang L. M. 2001. Rosiglitazone (BRL 49653) enhances insulin secretory response via phosphatidylinositol 3-kinase Pathway. Diabetes 50: 2598–2602. doi: 10.2337/diabetes.50.11.2598 [DOI] [PubMed] [Google Scholar]
- 22.Zoran D. L. 2010. Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin. North Am. Small Anim. Pract. 40: 221–239. doi: 10.1016/j.cvsm.2009.10.009 [DOI] [PubMed] [Google Scholar]