The N-terminal calmodulin-like domain of the human mitochondrial ATP-Mg/Pi carrier SCaMC1 was crystallized in the presence of Ca2+. X-ray diffraction data were collected to 2.9 Å resolution from crystals which belonged to space group P6222.
Keywords: mitochondrial ATP-Mg transporter, SCaMC, SLC25A24, calcium-sensing domain, conformation switch
Abstract
SCaMC is an ATP-Mg/Pi carrier protein located at the mitochondrial inner membrane. SCaMC has an unusual N-terminal Ca2+-binding domain (NTD) in addition to its characteristic six-helix transmembrane bundle. The NTD of human SCaMC1 (residues 1–193) was expressed and purified in order to study its role in Ca2+-regulated ATP-Mg/Pi transport mediated by its transmembrane domain. While Ca2+-bound NTD could be crystallized, the apo state resisted extensive crystallization trials. Selenomethionine-labeled Ca2+-bound NTD crystals, which belonged to space group P6222 with one molecule per asymmetric unit, diffracted X-rays to 2.9 Å resolution.
1. Introduction
Ca2+ is one of the most important secondary signaling messengers and is involved in a wide range of physiological events (Clapham, 2007 ▶). These include muscle contraction, permeability of ion channels, induction of mitochondrial permeability transition and apoptosis, RNA processing and many more (Clapham, 2007 ▶; Yang & Doublié, 2011 ▶). During the study of mitochondrial permeability transition, a related Ca2+-activated ATP-Mg/Pi exchange activity in mitochondria was first described (Nosek et al., 1990 ▶), and it was subsequently identified to be mediated by short calcium-binding mitochondrial carriers (SCaMCs; Chen, 2004 ▶; del Arco & Satrústegui, 2004 ▶; Fiermonte et al., 2004 ▶; Mashima et al., 2003 ▶).
SCaMCs belong to the mitochondrial carrier family consisting of structurally related transmembrane transporter proteins that shuttle important metabolites, nucleotides and cofactors across the mitochondrial inner membrane (Chen, Li et al., 2012 ▶; Klingenberg, 2009 ▶; Palmieri, 2008 ▶; Walker & Runswick, 1993 ▶). SCaMC has an unusual N-terminal domain (NTD) appended to the characteristic six transmembrane helices of mitochondrial carriers (Berardi et al., 2011 ▶; Pebay-Peyroula et al., 2003 ▶). Primary-sequence analysis revealed that the SCaMC NTD contains four EF-hand Ca2+-binding motifs (Chen, 2004 ▶; del Arco & Satrústegui, 2004 ▶; Fiermonte et al., 2004 ▶; Mashima et al., 2003 ▶) and has ∼50% sequence similarity to calmodulin (CaM), a well characterized Ca2+-sensor protein (Chagot & Chazin, 2011 ▶; Chou et al., 2001 ▶; Hoeflich & Ikura, 2002 ▶; Ikura et al., 1992 ▶; Kuboniwa et al., 1995 ▶). In coherence with the possession of a Ca2+-binding domain, SCaMC-mediated ATP-Mg/Pi exchange is inactive in the resting state (low Ca2+ concentration) and is stimulated by increased intracellular Ca2+ concentration (Amigo et al., 2013 ▶; Cavero et al., 2005 ▶; Traba et al., 2012 ▶). To better understand the functional role of SCaMC NTD in the Ca2+-mediated carrier activation, we set out to obtain structural information on the NTD in the presence and absence of Ca2+. Several paralogs of SCaMC have been identified in humans, including SCaMC-1/SLC25A24, SCaMC-2/SLC25A25, SCaMC-3/SLC25A23 (Bassi et al., 2005 ▶; del Arco & Satrústegui, 2004 ▶; Fiermonte et al., 2004 ▶; Mashima et al., 2003 ▶), SCaMC-1L (Amigo et al., 2012 ▶) and SCaMC-3L (Traba et al., 2009 ▶). In this report, we describe the preparation, crystallization and X-ray diffraction analysis of human SCaMC1 NTD.
2. Materials and methods
2.1. Cloning, expression and purification of human SCaMC1 NTD
A gene encoding the NTD (residues 1–193) of human SCaMC1 (UniProt ID Q6NUK1) was codon-optimized for expression in Escherichia coli, synthesized by GenScript and subsequently cloned into pET-21a expression vector (Novagen) using NdeI and XhoI restriction-enzyme sites. The NTD was transformed into E. coli BL21 (DE3) cells (New England Biolabs) and was expressed using autoinduction at 293 K (Studier, 2005 ▶). For purification of the NTD, the cells were lysed and the supernatant was passed through cobalt resin (Clontech; Chen, Gamache et al., 2012 ▶) pre-equilibrated with 20 mM HEPES–NaOH pH 7.4, 150 mM NaCl, 5 mM imidazole. The resin was washed in the same buffer with 20 mM imidazole. The protein was eluted with 200 mM imidazole and was then passed through a HiLoad 16/60 Superdex 75 size-exclusion column (GE Healthcare) in 20 mM HEPES–NaOH pH 7.0, 20 mM NaCl, 5 mM CaCl2. The apo form of NTD was obtained by extensive dialysis against 20 mM HEPES–NaOH pH 7.0, 20 mM NaCl, 20 mM EDTA, 5 mM EGTA three times (12 h each) followed by final dialysis against 20 mM HEPES pH 7.0, 20 mM NaCl, 5 mM EDTA. Ca2+-bound and apo NTD were concentrated to 10 mg ml−1 using an Amicon YM-10 concentrator (Millipore) and were immediately used for crystallization. A total of 5 mg of purified NTD was obtained from a 1 l culture. Selenomethionine-labeled protein was prepared using a previously published protocol (Doublié, 2007 ▶) and was purified as described above.
2.2. Crystallization, data collection and processing
A Mosquito HTS crystallization robot (TTP LabTech) was used to set up 96-well sitting-drop plates (TTP LabTech) by mixing 200 nl NTD solution with 200 nl crystallization solution from the Wizard III and IV crystallization screens (Emerald BioSystems). For Ca2+-bound NTD, crystals were identified in eight of the 96 conditions. Condition No. 56 [100 mM Tris pH 8.5, 2 M Li2SO4, 2%(w/v) PEG 400], which gave the best initial crystal morphology, was further optimized in a 24-well VDX plate (Hampton Research) by performing several rounds of hanging-drop experiments in which the pH, precipitant concentration and additives were varied. Crystals appeared in 2 d and grew to 0.1 mm in size within one week at 293 K. Crystals were cryoprotected during the course of growth; no further procedure was performed prior to cryocooling in liquid nitrogen (Yang, Gilmartin et al., 2011 ▶). For apo-form NTD, several commercial crystallization screen kits, Crystal Screen HT, Index HT, PEG/Ion HT, SaltRx (Hampton Research), Wizard I and II and Wizard III and IV (Emerald BioSystems), were tested. However, no crystals were obtained before the plates completely dried out.
A complete multiple-wavelength anomalous diffraction (MAD) data set was collected on Advanced Photon Source beamline 23-ID-D at Argonne National Laboratory using a MAR 300 CCD detector. Three wavelengths were used for MAD data-set collection: 0.9794 Å (peak), 0.9795 Å (inflection) and 0.9494 Å (remote). The diffraction data were indexed, integrated and scaled using the HKL-2000 suite of programs (Otwinowski & Minor, 1997 ▶) installed, configured and provided by SBGrid (Morin et al., 2013 ▶). The NTD structure was solved using phenix.autosol (Adams et al., 2010 ▶). Crystallographic statistics are shown in Table 1 ▶.
Table 1. X-ray data-collection and processing statistics.
Values in parentheses are for the outermost resolution shell.
| SeMet peak | SeMet inflection | SeMet remote | |
|---|---|---|---|
| Data collection | |||
| Wavelength (Å) | 0.9794 | 0.9795 | 0.9494 |
| Resolution (Å) | 40–2.90 (2.98–2.90) | 40–2.92 (3.03–2.92) | 40–3.00 (3.10–3.00) |
| Space group | P6222 | P6222 | P6222 |
| Unit-cell parameters | |||
| a = b (Å) | 74.480 | 74.489 | 74.503 |
| c (Å) | 173.64 | 173.65 | 173.71 |
| α = β (°) | 90 | 90 | 90 |
| γ (°) | 120 | 120 | 120 |
| Total reflections | 260454 (20594) | 254334 (28333) | 235619 (23782) |
| Unique reflections | 11865 (886) | 11613 (1220) | 10726 (1023) |
| Multiplicity | 21.6 (23.2) | 21.9 (23.2) | 21.9 (23.2) |
| 〈I/σ(I)〉 | 26.89 (5.09) | 27.95 (5.74) | 27.22 (5.69) |
| Completeness (%) | 99.1 (100) | 99.0 (100) | 99.2 (100) |
| R merge † (%) | 8.0 (90.8) | 7.9 (80.7) | 8.1 (86.5) |
| Phasing | |||
| No. of SeMet sites | 4 | ||
| Figure of merit (phenix.autosol) | 0.58 | ||
R
merge = 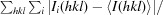
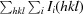 , where Ii(hkl) is the observed intensity and 〈I(hkl)〉 is the average intensity for multiple measurements.
, where Ii(hkl) is the observed intensity and 〈I(hkl)〉 is the average intensity for multiple measurements.
3. Results and discussion
3.1. Crystallization of Ca2+-bound SCaMC1 NTD
Full-length human SCaMC1 (also referred to as SLC25A24) consists of 477 residues. A sequence analysis of SCaMC1 using the MemBrain transmembrane protein structure prediction server (Shen & Chou, 2008 ▶) and sequence alignment with other mitochondrial carrier proteins suggested that the transmembrane domain is composed of residues 194–477. The NTD composed of residues 1–193 and a C-terminal His6 tag with an expected molecular weight of 22.9 kDa was constructed in order to dissect its functional role in Ca2+ sensing. After cobalt resin affinity purification and size-exclusion chromatography, the NTD migrated on SDS–PAGE as a single band with a molecular weight of ∼23 kDa (Fig. 1 ▶). For the Ca2+-bound NTD, crystals were obtained in eight of the 96 conditions screened, more specifically Nos. 1, 3, 5, 10, 32, 44, 56 and 60 from the Wizard III and IV screens (Emerald BioSystems). After several rounds of optimization based on condition No. 56, large single crystals (0.1 × 0.03 × 0.01 mm; Fig. 2 ▶) were obtained from a hanging drop consisting of 1 µl Ca2+-bound NTD solution (10 mg ml−1) and 1 µl reservoir solution consisting of 50 mM Tris pH 7.8, 2 M Li2SO4, 4%(w/v) PEG 400 equilibrated against 500 µl reservoir solution at 293 K. Crystals appeared in 2 d and grew to full size in one week. As indicated by the diffraction pattern (Fig. 3 ▶), 2 M Li2SO4 in the reservoir was sufficient to cryoprotect the crystals, similar to previous observations using 2 M NaCl as reservoir solution (Yang, Coseno et al., 2011 ▶; Yang, Faucher et al., 2011 ▶; Yang et al., 2010 ▶, 2013 ▶). Thus, no additional cryoprotectant solution was needed. Attempts to crystallize NTD in the absence of Ca2+ were unsuccessful. In synergy with the success rate of crystallization, the Ca2+-bound NTD is in a compact, more rigid form, whereas the apo NTD is in a flexible, less structured form in solution, as demonstrated by studying the dynamics of NTD using nuclear magnetic resonance (Yang et al., 2014 ▶).
Figure 1.
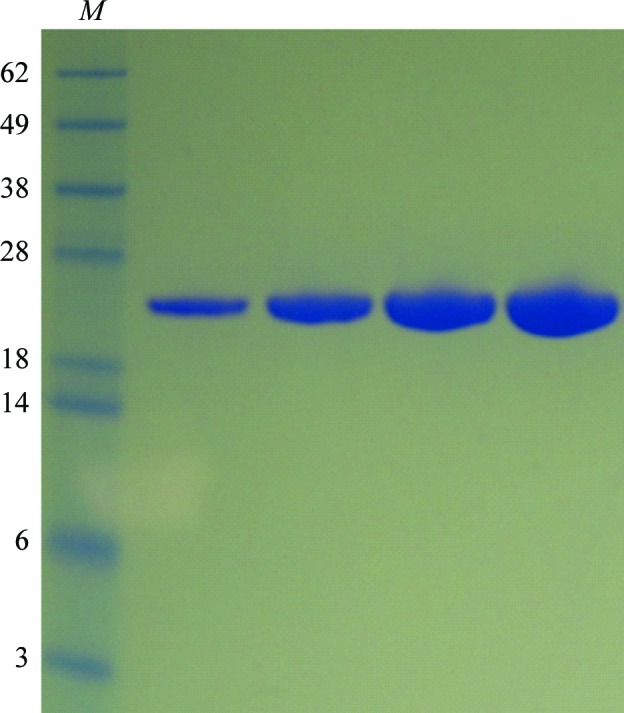
SDS–PAGE of the purified NTD loaded at increased protein concentrations. Lane M contains molecular-mass marker (labelled in kDa).
Figure 2.
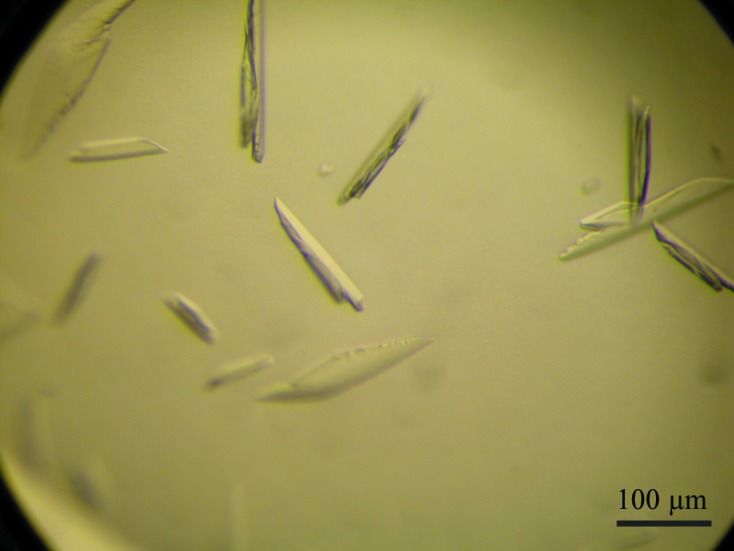
Crystal of Ca2+-bound human SCaMC1 NTD (residues 1–193). The dimensions of the crystals are about 0.1 × 0.03 × 0.01 mm.
Figure 3.
A representative diffraction image of the NTD crystal with a 2.9 Å resolution limit.
3.2. Initial X-ray diffraction analysis
Three complete data sets at different wavelengths were collected using a selenomethionine-labeled crystal that diffracted to 2.9 Å resolution. The data were indexed in space group P6222, with unit-cell parameters a = b = 74.48, c = 173.637 Å, α = β = 90, γ = 120°. The calculated Matthews coefficient (V M) of 3.20 Å3 Da−1, with a corresponding solvent content of 61%, suggests the presence of one molecule per asymmetric unit (Matthews, 1968 ▶). The NTD structure was solved by MAD using phenix.autosol (Adams et al., 2010 ▶). All four selenomethionine sites were identified. After density modification, 134 of 193 residues were built using phenix.autobuild (Adams et al., 2010 ▶). Further model building and structural analysis will be reported in a separate paper (Yang et al., 2014 ▶).
Acknowledgments
We thank members of the Chou laboratory for technical assistance and insightful discussions and Dr Yu Chen (Harvard Medical School) for X-ray data collection. This work is based upon research conducted at the Advanced Photon Source (Northeastern Collaborative Access Team beamlines). SB is the recipient of an Erwin Schrödinger postdoctoral fellowship from the Austrian Science Fund (FWF, J3251). This work was supported by NIH grant No. GM094608 (to JJC).
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Amigo, I., Traba, J., González-Barroso, M. M., Rueda, C. B., Fernández, M., Rial, E., Sánchez, A., Satrústegui, J. & Del Arco, A. (2013). J. Biol. Chem. 288, 7791–7802. [DOI] [PMC free article] [PubMed]
- Amigo, I., Traba, J., Satrústegui, J. & del Arco, A. (2012). PLoS One, 7, e40470. [DOI] [PMC free article] [PubMed]
- Arco, A. del & Satrústegui, J. (2004). J. Biol. Chem. 279, 24701–24713. [DOI] [PubMed]
- Bassi, M. T., Manzoni, M., Bresciani, R., Pizzo, M. T., Della Monica, A., Barlati, S., Monti, E. & Borsani, G. (2005). Gene, 345, 173–182. [DOI] [PubMed]
- Berardi, M. J., Shih, W. M., Harrison, S. C. & Chou, J. J. (2011). Nature (London), 476, 109–113. [DOI] [PMC free article] [PubMed]
- Cavero, S., Traba, J., Del Arco, A. & Satrústegui, J. (2005). Biochem. J. 392, 537–544. [DOI] [PMC free article] [PubMed]
- Chagot, B. & Chazin, W. J. (2011). J. Mol. Biol. 406, 106–119. [DOI] [PMC free article] [PubMed]
- Chen, W., Gamache, E., Richardson, D., Du, Z. & Wang, C. (2012). Protein Expr. Purif. 81, 11–17. [DOI] [PubMed]
- Chen, W., Li, L., Du, Z., Liu, J., Reitter, J. N., Mills, K. V., Linhardt, R. J. & Wang, C. (2012). J. Am. Chem. Soc. 134, 2500–2503. [DOI] [PMC free article] [PubMed]
- Chen, X. J. (2004). Genetics, 167, 607–617. [DOI] [PMC free article] [PubMed]
- Chou, J. J., Li, S., Klee, C. B. & Bax, A. (2001). Nature Struct. Biol. 8, 990–997. [DOI] [PubMed]
- Clapham, D. E. (2007). Cell, 131, 1047–1058. [DOI] [PubMed]
- Doublié, S. (2007). Methods Mol. Biol. 363, 91–108. [DOI] [PubMed]
- Fiermonte, G., De Leonardis, F., Todisco, S., Palmieri, L., Lasorsa, F. M. & Palmieri, F. (2004). J. Biol. Chem. 279, 30722–30730. [DOI] [PubMed]
- Hoeflich, K. P. & Ikura, M. (2002). Cell, 108, 739–742. [DOI] [PubMed]
- Ikura, M., Clore, G. M., Gronenborn, A. M., Zhu, G., Klee, C. B. & Bax, A. (1992). Science, 256, 632–638. [DOI] [PubMed]
- Klingenberg, M. (2009). Biochim. Biophys. Acta, 1788, 2048–2058. [DOI] [PubMed]
- Kuboniwa, H., Tjandra, N., Grzesiek, S., Ren, H., Klee, C. B. & Bax, A. (1995). Nature Struct. Biol. 2, 768–776. [DOI] [PubMed]
- Mashima, H., Ueda, N., Ohno, H., Suzuki, J., Ohnishi, H., Yasuda, H., Tsuchida, T., Kanamaru, C., Makita, N., Iiri, T., Omata, M. & Kojima, I. (2003). J. Biol. Chem. 278, 9520–9527. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Morin, A., Eisenbraun, B., Key, J., Sanschagrin, P. C., Timony, M. A., Ottaviano, M. & Sliz, P. (2013). Elife, 2, e01456. [DOI] [PMC free article] [PubMed]
- Nosek, M. T., Dransfield, D. T. & Aprille, J. R. (1990). J. Biol. Chem. 265, 8444–8450. [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Palmieri, F. (2008). Biochim. Biophys. Acta, 1777, 564–578. [DOI] [PubMed]
- Pebay-Peyroula, E., Dahout-Gonzalez, C., Kahn, R., Trézéguet, V., Lauquin, G. J. & Brandolin, G. (2003). Nature (London), 426, 39–44. [DOI] [PubMed]
- Shen, H. & Chou, J. J. (2008). PLoS One, 3, e2399. [DOI] [PMC free article] [PubMed]
- Studier, F. W. (2005). Protein Expr. Purif. 41, 207–234. [DOI] [PubMed]
- Traba, J., Del Arco, A., Duchen, M. R., Szabadkai, G. & Satrústegui, J. (2012). Cell Death Differ. 19, 650–660. [DOI] [PMC free article] [PubMed]
- Traba, J., Satrústegui, J. & del Arco, A. (2009). Biochem. J. 418, 125–133. [DOI] [PubMed]
- Walker, J. E. & Runswick, M. J. (1993). J. Bioenerg. Biomembr. 25, 435–446. [DOI] [PubMed]
- Yang, Q., Brüschweiler, S. & Chou, J. J. (2014). Structure, 10.1016/j.str.2013.10.018.
- Yang, Q., Coseno, M., Gilmartin, G. M. & Doublié, S. (2011). Structure, 19, 368–377. [DOI] [PMC free article] [PubMed]
- Yang, Q. & Doublié, S. (2011). Wiley Interdiscip. Rev. RNA, 2, 732–747. [DOI] [PMC free article] [PubMed]
- Yang, Q., Faucher, F., Coseno, M., Heckman, J. & Doublié, S. (2011). Acta Cryst. F67, 241–244. [DOI] [PMC free article] [PubMed]
- Yang, Q., Gilmartin, G. M. & Doublié, S. (2010). Proc. Natl Acad. Sci. USA, 107, 10062–10067. [DOI] [PMC free article] [PubMed]
- Yang, Q., Gilmartin, G. M. & Doublié, S. (2011). RNA Biol. 8, 748–753. [DOI] [PMC free article] [PubMed]
- Yang, Q., Nausch, L. W. M., Martin, G., Keller, W. & Doublié, S. (2013). J. Mol. Biol., 10.1016/j.jmb.2013.09.025. [DOI] [PMC free article] [PubMed]



