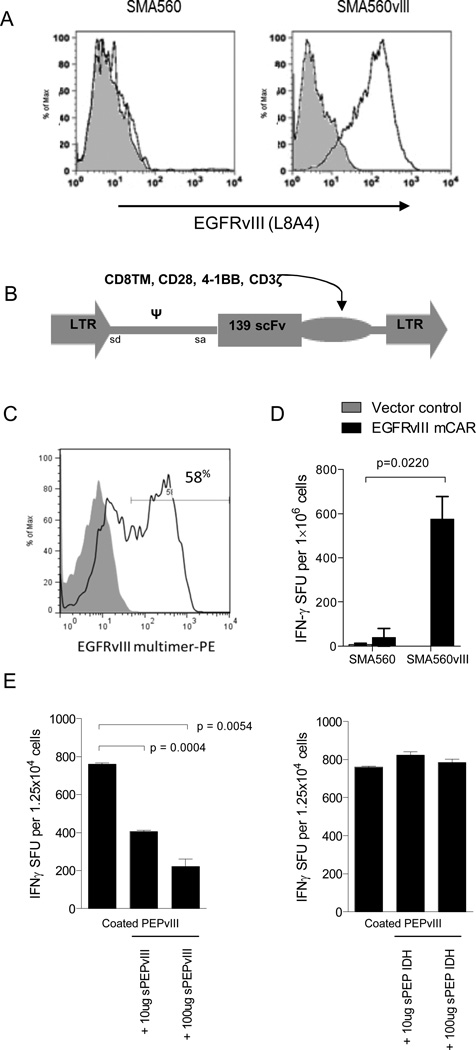
Figure 1. EGFRvIII mCAR retroviral gene delivery to murine T cells confers antigen-specific activity.
(A) VM/Dk-derived glioma SMA560vIII shows EGFRvIII expression compared with SMA560 by surface antibody staining (L8A4) and flow cytometry; grey represents negative staining control. (B) The EGFRvIII mCAR MSGV1 retroviral vector contains the human anti-EGFRvIII scFv 139, in tandem with murine CD8TM, CD28, 4-1BB, and CD3ζ intracellular regions. EGFRvIII mCAR MSGV1 retrovirus containing supernatant was generated by transient transfection of HEK 293T cells with the corresponding retroviral vectors and the helper pCL-Eco plasmid. Supernatants harvested 48h after transfection were used to transduce previously ConA activated murine VM/Dk splenocytes. (C) Retroviral mCAR gene expression was evaluated by flow cyometric analysis 3 days after transduction; grey represents negative staining control. (D) Antigen specificity of EGFRvIII mCAR transduced and vector control (MSGV1-GFP) T cells was evaluated against EGFRvIII-expressing SMA560 cells (SMA560vIII) or the parental EGFRvIIINEG line (SMA560) by IFNγ ELISpot assay. Statistical analysis was performed by two-way ANOVA. Horizontal bar represents a statistical significance of P < 0.05 (E) Soluble EGFRvIII peptide (PEPvIII) at 50 μg/mL was used to coat tissue culture plates prior to addition of EGFRvIII mCAR or GFP control T cells. Cells were cultured 18h in the presence of increasing dosages of soluble PEPvIII or irrelevant peptide (IDH) and subjected to IFNγ ELISpot. Statistical analysis was performed by unpaired t test comparing groups defined by EGFRvIII target expression. Horizontal bar represents a statistical significance of P < 0.05. Experiments are representative of two independent repeats with similar results.