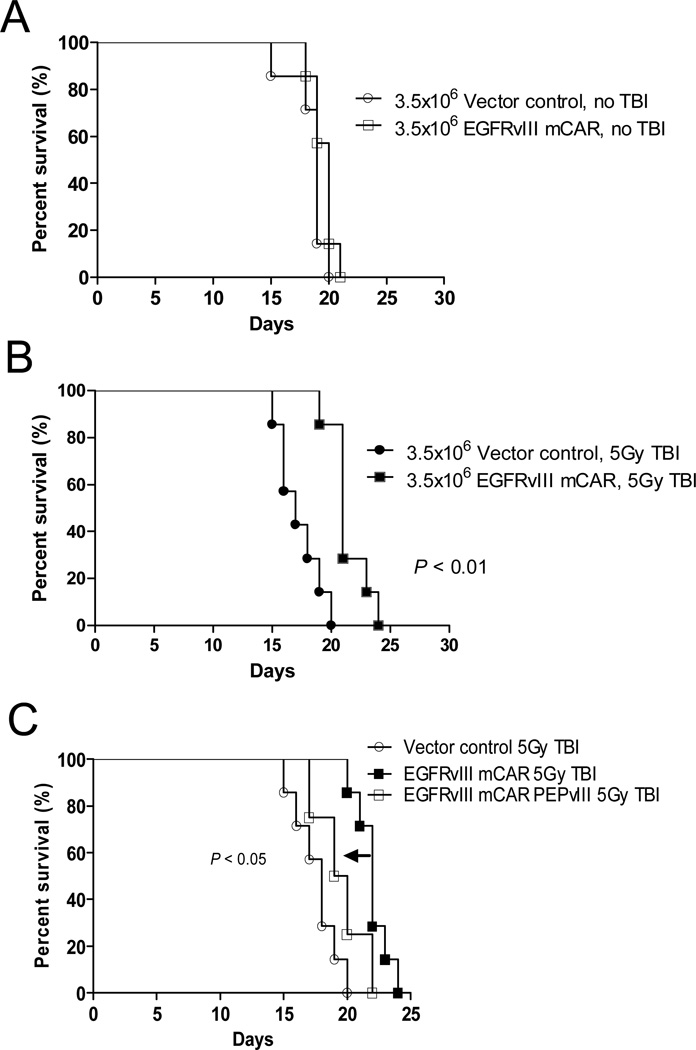
Figure 2. EGFRvIII mCAR T-cell therapy against intracerebral tumors requires lymphodepletive host-conditioning for efficacy, and can be abrogated by soluble peptide administration.
To evaluate the impact of lymphopenia on the antitumor efficacy of EGFRvIII mCAR T cells, VM/Dk mice (8 per group) with 3-day established intracerebral SMA560vIII tumors were left untreated (A, no TBI) or irradiated (B, 5Gy TBI) prior to intravenous infusion with 3.5×106 vector control (MSGV1-GFP) or EGFRvIII mCAR T cells. (C) To evaluate whether a short peptide corresponding to the antigenic epitope expressed on target cells could be applied to competitively inhibit and reduce the functional activity of EGFRvIII mCAR T cells in vivo, mice with 3 day implanted SMA560vIII treated as in (B) above, were injected with 100 μg PEPvIII or saline 4h following mCAR T cell infusion intravenously, followed by a second injection 10 days later. Mice were monitored for morbidity end points approved by the Duke University IACUC and sacrificed when end points were met. Survival analysis was performed using the Log-Rank (Mantel Cox) test. Statistical significance was determined at P < 0.05.