Abstract
Mobilized peripheral blood has become the predominant stem cell source for allogeneic hematopoietic cell transplantation. In this retrospective single center study of 442 patients with hematologic malignancies, we analyzed prognostic factors for long-term survival after peripheral blood stem cell transplantation from HLA-matched related or unrelated donors. To account for disease/status heterogeneity, patients were risk-stratified according to the Disease Risk Index. Five-year overall survival was similar after transplants with matched related and unrelated donors (45% and 46%, respectively; P=0.49). Because donor age ≥60 years impacted outcome during model building, we further considered 3 groups of donors: matched unrelated (aged <60 years by definition), matched related aged <60 years and matched related aged ≥60 years. In multivariate analysis, the donor type/age group and the graft CD34+ and CD3+ cell doses impacted long-term survival. Compared with matched unrelated donor transplant, transplant from matched related donor <60 years resulted in similar long-term survival (P=0.67) while transplant from matched related donor ≥60 years was associated with higher risks for late mortality (hazard ratio (HR) 4.41; P=0.006) and treatment failure (HR: 6.33; P=0.009). Lower mortality risks were observed after transplant with CD34+ cell dose more than 4.5×106/kg (HR: 0.56; P=0.002) and CD3+ cell dose more than 3×108/kg (HR: 0.61; P=0.01). The Disease Risk Index failed to predict survival. We built an “adapted Disease Risk Index” by modifying risks for myeloproliferative neoplasms and multiple myeloma that improved stratification ability for progression-free survival (P=0.04) but not for overall survival (P=0.82).
Introduction
Over the past decade, mobilized peripheral blood (PB) has progressively overtaken bone marrow as source of stem cells for allogeneic hematopoietic stem cell transplantation (HSCT).1 This was supported by evidence of faster engraftment,2–5 decreased relapse in high-risk patients6 and, for some authors, better survival after PB-HSCT. However, higher risks for chronic graft-versus-host disease (GVHD) associated with PB-HSCT are of particular concern. Despite this, the number of PB-HSCT is still increasing.7
Human leukocyte antigen (HLA)-matched unrelated donor (MUD) is now a widely accepted alternative donor source when no suitable HLA-matched related donor (MRD) is available. Outcome after MUD-HSCT has dramatically improved over the past 20 years, mainly due to the advent of high resolution allelic level HLA typing techniques.8 Most US centers consider donor/recipient allelic matching at HLA-A, -B, -C and -DRB1 loci for MUD selection (8/8 MUD) while many European centers also further search for allelic identity at the HLA-DQB1 locus (10/10 MUD). Several prospective and large retrospective studies have reported current similar survival after MUD-HSCT compared to MRD-HSCT for patients with hematologic malignancies.9–13 However, few studies have considered PB as the sole stem cell source and 10/10 allelic level HLA-matched donor/recipient pairs for MUD-HSCT.
Regardless of donor type, other pre-transplant factors may impact survival after PB-HSCT. Identification of clinical predictors of long-term success of PB-HSCT is important for patient counseling and clinical trial design. Recently, Armand et al. have proposed a new tool for risk-stratifying patients with respect to overall survival (OS) and progression-free survival (PFS) on the basis of disease diagnoses and stages at the time of HSCT: the Disease Risk Index (DRI).14 This index was applicable regardless of conditioning intensity. Although Armand et al. validated this index in an independent cohort, DRI needs to be further tested in other independent populations.
Here, we report a large single center retrospective study of PB-HSCT with MRD or 10/10 MUD in patients with hematologic malignancies. To account for disease/status heterogeneity, patients were stratified for disease risk according to the DRI. The aims of this study were: 1) to evaluate how donor type (MRD vs. MUD) and other pre-transplant factors may predict for long-term OS and PFS after PB-HSCT; and 2) to evaluate DRI as a predictor for OS and PFS.
Methods
Patients
We performed a retrospective analysis of all consecutive patients with hematologic malignancies who underwent a first PB-HSCT from either MRD or MUD at Saint-Louis Hospital (Paris, France) between January 2000 and December 2010. According to institutional guidelines, MRD was considered as the first donor choice and search for MUD was only undertaken if no suitable MRD was identified. For MUD PB-HSCT, only donor/recipient pairs matched at the allelic level at HLA-A, -B, -C, -DRB1 and -DQB1 loci (10/10 HLA-matched) were included. Data concerning pre-transplant characteristics and transplant outcomes were extracted from our transplantation database. To assess for pre-transplant comorbidities, the hematopoietic cell transplantation-specific comorbidity index (HCT-CI)15 was retrospectively calculated for patients transplanted between 2006 and 2010 (Online Supplementary Methods). All patients provided written informed consent for use of protected health data for research, in accordance with the Declaration of Helsinki.
Disease risk at transplant
Disease risk was retrospectively assessed according to disease diagnosis and disease stage at the time of HSCT, using the DRI.14 In the original paper, diseases underrepresented in the training cohort were randomly assigned into the intermediate disease type risk category (first step of DRI building). These included myelo-proliferative neoplasms (MPN) and multiple myeloma (MM). We, therefore, built an adapted DRI in which both of these diseases were assigned risks according to their observed outcomes and we compared it with the original DRI.
Definitions and study end points
Definitions for transplant modalities and outcomes are available in the Online Supplementary Methods. End points of the study were OS and PFS. To assess long-term outcome after HSCT, we also studied relapse, non-relapse mortality (NRM) and chronic GVHD.
Statistical methods
Characteristics of patients transplanted from MRD or MUD were compared using Wilcoxon rank sum tests and Fisher exact tests. Outcomes were censored at 60 months, given the study follow up. Relapse and NRM were considered to be mutually competing risks. OS was estimated using the Kaplan-Meier product-limit estimator. For competing risks analyses, cumulative incidence functions were estimated using the usual methodology.16
Factors associated with outcomes were analyzed using proportional hazards models for the cause-specific hazard (chronic GVHD, relapse and NRM)17 and Cox proportional hazards models (PFS and OS). Tested variables (only pre-transplant parameters) are listed in the Online Supplementary Methods. The proportional hazards assumption was checked by examination of Schoenfeld residuals and the Grambsch and Therneau lack-of-fit test.18 For multivariate analysis, all variables achieving P<0.25 in univariate analysis were considered. No backward variable elimination procedure was used. The usual ‘rule of thumb’ of 10 events per variable was not enforced but we ensured the models had no less than 5 events per variable as this has been shown to yield similar properties.19 All tests were two-sided and P≤0.05 was considered significant.
Stratification ability of DRIs was evaluated by the C-statistic. Comparison of DRIs involved testing of null differences between C-indexes, integrated discrimination improvement index (IDI) and continuous net reclassification index (NRI).22–25
Analyses were performed using the R-statistical software version 2.15.0.26
Results
Study patients
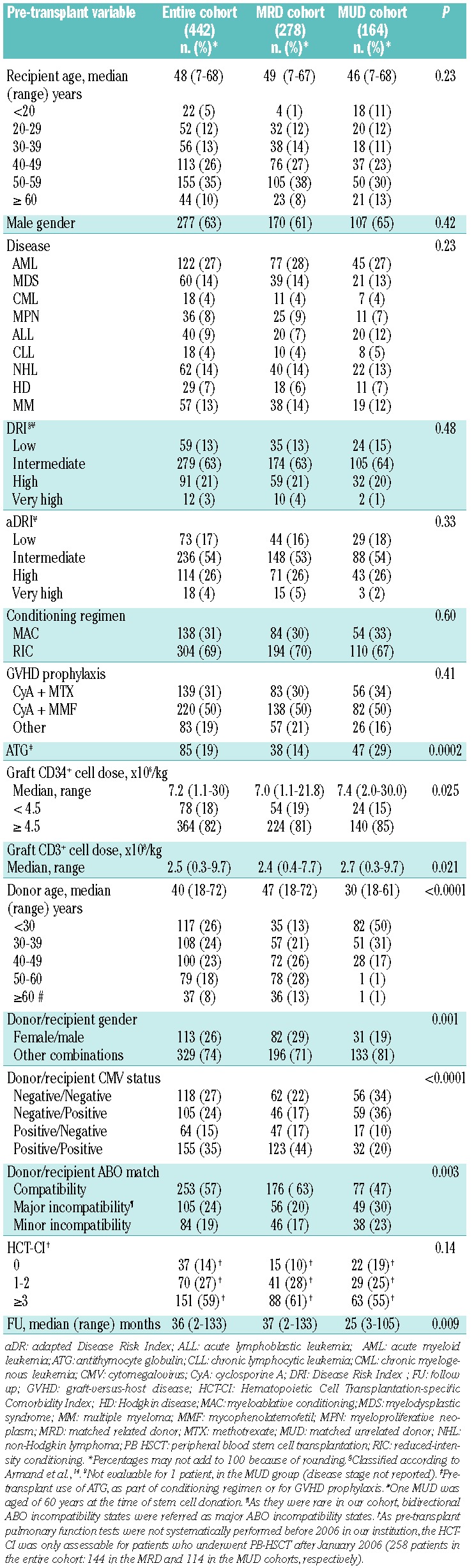
A total of 442 patients were included. Base-line characteristics are summarized in Table 1. Overall, 278 patients were transplanted from MRD and 164 from MUD. MUD and MRD cohorts were balanced for patient age, disease distribution, DRI and intensity of the conditioning regimen.
Table 1.
Base-line patients’, disease and transplant characteristics
Median follow-up time after transplant was 36 months with 25% of patients being followed for at least 60 months. The 5-year OS was 45% (95% confidence interval (CI): 35–57) and 46% (95%CI: 39–54) (P=0.49); PFS was 43% (95%CI: 34–54) and 39% (95%CI: 32–46) (P=0.72); and NRM was 33% (95%CI: 24–42) and 22% (95%CI: 17–27) (P=0.071) after MUD and MRD PB-HSCT, respectively. In the MUD cohort, the most frequent primary cause of death was HSCT-related (65%) while relapse accounted for 31%. In the MRD cohort, relapse accounted for 50% of primary cause of death. The 5-year cumulative incidence (CIf) of relapse was lower after MUD transplant (24%, 95%CI: 17–32%) than after MRD transplant (39%, 95%CI: 32–46%) (P=0.038). The 5-year CIf of chronic GVHD was 59% (95% CI: 50–67%) and 58% (95%CI: 51–64%) after MUD and MRD PB-HSCT, respectively (P=0.26).
Impact of pre-transplant factors on overall and progression-free survival
Univariate analysis (Online Supplementary Table S1)
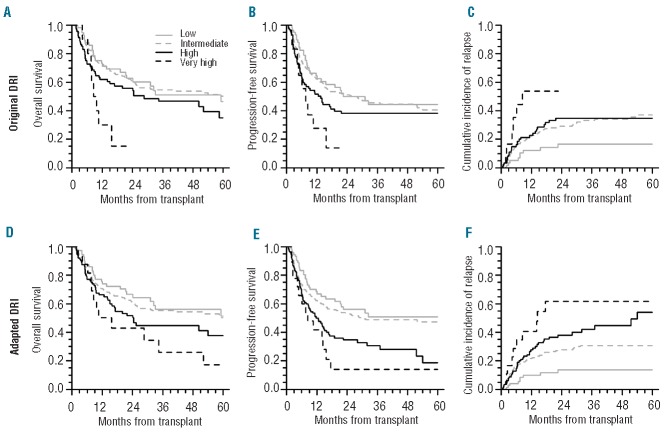
Donor type (MRD vs. MUD) was not associated with survival. Factors that impacted survival were older donor age (≥60 years), graft CD34+ and CD3+ cell doses, DRI and recipient age. As almost all donors ≥60 years old were MRD (except 1 MUD aged 60 years), 3 groups of donors according to type and age (MUD, MRD<60y and MRD≥60y) were further defined. Patients transplanted with MRD≥60y experienced low 5-year OS (6%, standard error 6%) (Figure 1A) and relapse accounted for 68% of their cause of death. Characteristics of patients transplanted with MRD≥60y are provided in the Online Supplementary Table S2. DRI did not fully stratify patients for OS and PFS as no significant difference was observed for patients with low, intermediate and high DRI (Figure 2A and B).
Figure 1.
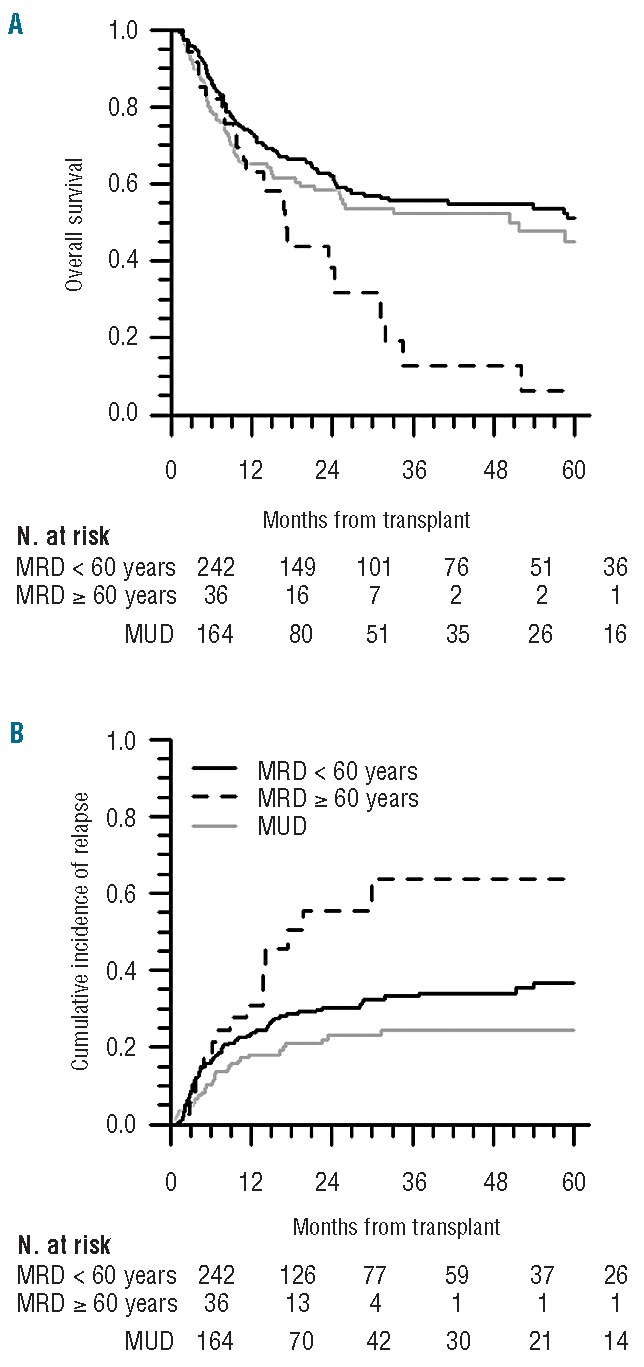
Outcomes according to donor type/age groups. OS (A) and cumulative incidence of relapse (B). For relapse, patients at risk were patients alive and relapse-free at each time.
Figure 2.
Outcomes according to DRI (A, B, C) and aDRI (D, E, F). OS (A, D), PFS (B, E) and cumulative incidence of relapse (C, F).
Multivariate analysis
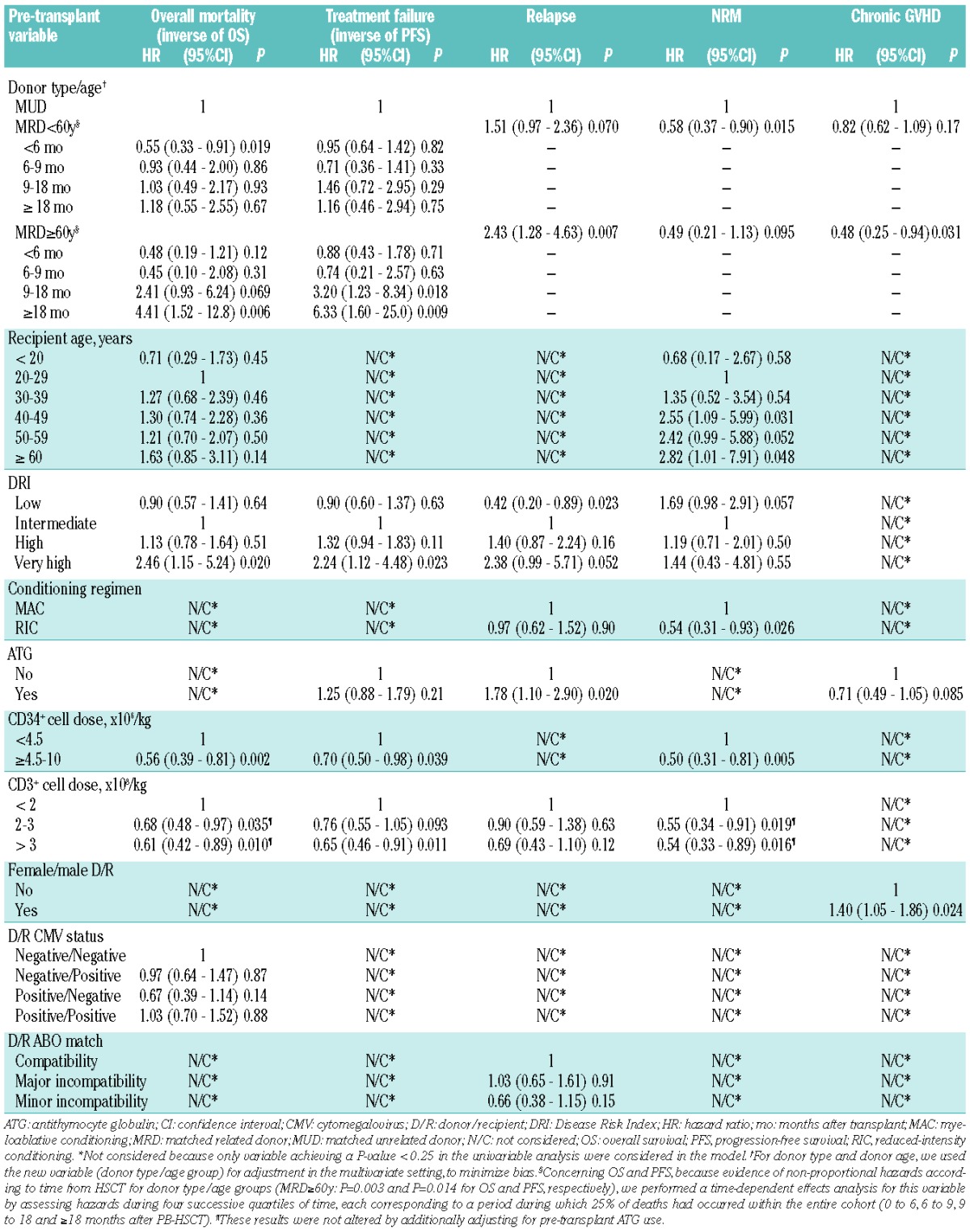
Variables significantly impacting OS and PFS were the donor type/age group and graft CD34+ and CD3+ cell doses (Table 2). Compared with MUD-PB-HSCT, PB-HSCT with MRD<60y resulted in similar risks for late mortality and treatment failure while PB-HSCT with MRD≥60y was associated with higher risks for late overall mortality and treatment failure (from 18 and 9 months post-transplant, respectively). Regardless of donor source, high graft CD34+ and CD3+ cell doses significantly predicted lower risks for mortality and treatment failure. DRI only predicted survival for patients within the very high risk category. Higher recipient age was associated with increased hazard ratio for mortality risks, but the correlation did not remain significant.
Table 2.
Multivariate analysis of pre-transplant factors associated with outcomes after PB-HSCT.
Relapse, non-relapse mortality and chronic graft-versus-host disease
We further evaluated how these pre-transplant predictors of OS and PFS impacted other long-term outcomes (Table 2 and Online Supplementary Table S1).
Donor type and age
When considering donor type/age groups, we observed that patients transplanted with MRD≥60y experienced a high relapse rate (Figure 1B). In multivariate analysis, PB-HSCT with MRD≥60y resulted in higher risks for relapse, lower risks for chronic GVHD and a trend of lower NRM as compared with MUD PB-HSCT.
Graft cell doses
In both univariate and multivariate analyses, high CD34+ and CD3+ cell doses predicted lower NRM.
DRI
DRI stratified relapse for patients with low and very high DRI (Figure 2C) in univariate analysis but only for patients with low DRI in multivariate analysis.
Subgroups analyses
Comorbidity
The HCT-CI15 was calculated for 258 patients (86% of the patients transplanted after January 2006). We built a multivariate model for OS and PFS adjusted for HCT-CI in this subgroup (Online Supplementary Table S3). The donor type/age group remained significant for survival prediction. High CD34+ and CD3+ cell doses were associated with a trend of higher OS and PFS. The DRI only predicted survival for patients with very high DRI. The HCT-CI was not associated with survival in this subpopulation.
Older patients
The majority of patients transplanted with MRD≥60 were ≥50 years old (30 of 36 patients) and were older than those transplanted from MUD or MRD<60y. We built a multivariate model for survival in a selected subgroup of 199 patients aged ≥50 years (71 patients transplanted with MUD, 98 with MRD<60 and 30 with MRD≥60) (Online Supplementary Table S3). As compared with MUD transplant, PB-HSCT with MRD≥60y was still associated with significantly higher risks for late treatment failure and a trend of higher risks for late overall mortality.
Adapted Disease Risk Index
In our study, Disease Risk Index was not fully predictive of either OS or PFS (Table 2, Figure 2A and B and Online Supplementary Table S3). In the original DRI publication, MPN and MM were randomly assigned into the intermediate disease type risk category.14 When assessing both diseases, we observed that MPN and MM patients had respectively better and worse outcomes than patients assigned to the intermediate disease type risk category (Online Supplementary Figure S1). Therefore, we constructed an “adapted DRI (aDRI)” in which MPN and MM were respectively assigned to the low-risk category and to the high-risk category for disease type (first step of DRI assessment).
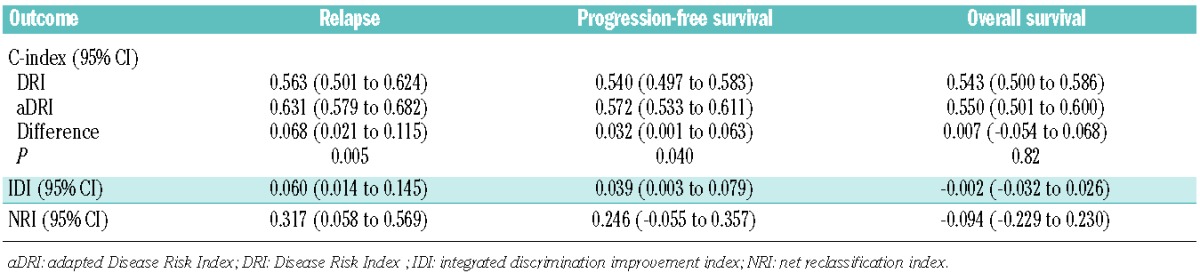
OS, PFS and cumulative incidence of relapse stratified by aDRI are shown in Figure 2D–F. In our multivariate model, aDRI successfully stratified patients for relapse (P<0.05), correlated with PFS for patients with intermediate, high and very high aDRI (P<0.05), but was not predictive for OS (Online Supplementary Table S4). Stratification ability of DRI and aDRI for OS, PFS and relapse were finally assessed (Table 3). Compared with DRI, aDRI better stratified relapse and PFS but not OS.
Table 3.
C-statistics for DRI and aDRI, and reclassification indexes comparing the stratification ability of aDRI to DRI.
Based on these results, previous multivariate analyses were verified using aDRI instead of DRI as candidate effects for adjustment. Similar correlations between risk factors and outcomes were observed as those reported above (data not shown).
Discussion
Identification of clinical predictors of long-term survival after HLA-matched PB-HSCT is a current major concern. Here, we reported a 10-year single center retrospective analysis of long-term OS and PFS after PB-HSCT from MRD or 10/10 MUD in patients with hematologic malignancies.
In accordance with previous studies reporting various graft sources,9–13 we observed similar 5-year OS after transplant from MUD and MRD with PB as the unique stem cell source. By further considering MRD age, we observed that PB-HSCT from MUD and MRD<60y indeed resulted in similar long-term survival, whereas PB-HSCT with MRD≥60y was associated with notably poor long-term OS and PFS.
Though it has to be interpreted with caution due to small sample size, the poor long-term survival we observed for patients transplanted with MRD≥60y is intriguing. Several factors may have contributed to their lower survival as compared with patients transplanted from MUD. 1) Patients transplanted from MRD≥60y were older and we could not formally exclude that their older age might not have contributed to their lower OS and PFS. However, in a subgroup analysis of selected older recipients (≥50 years old), PB-HSCT from MRD≥60y remained associated with lower long-term survival. 2) Because they were older, patients transplanted with MRD≥60y might also have heavier comorbidities. However, transplant from MRD≥60y remained associated with lower survival after adjustment for HCT-CI in a sub-analysis of patients for whom this index was assessable. 3) A significant proportion of patients transplanted with MRD≥60y fell into the high or very high (a)DRI categories. However, higher risks for mortality were still observed after MRD≥60y-transplant after adjustment for DRI or aDRI in multivariate analysis. 4) Lower CD34+ cell doses were collected from MRD≥60y. As CD34+ graft cell dose was associated with survival, this might have contributed to the lower OS and PFS observed after transplant with MRD≥60y.
The main cause of death of MRD≥60y recipients was relapse and relapse incidence was higher for these patients compared with MUD recipients. Lower risks for chronic GVHD were also observed after MRD≥60y-transplant compared with MUD-transplant. These results could not be explained by an ATG effect as MRD≥60y recipients did not receive ATG more frequently than MUD recipients, nor by the frequent use of RIC for patients transplanted with MRD≥60y as the intensity of the conditioning was not associated with these outcomes. Because of its potential anti-tumoral effect, the low chronic GVHD incidence experienced by MRD≥60y recipients might have contributed to the high relapse rate. Moreover, the high relapse rate and the low incidence of chronic GVHD after transplant with MRD≥60y may also suggest lower allore-activity of grafts from older MRD. This can be supported by some experimental data having shown a decline of T-cell allogeneic reactivity27 and an increase in the number of circulating tolerogenic regulatory T cells with advanced age.
Several studies have reported adverse impact of older donor age on OS after HSCT.30–36 However, few studies have assessed the combined effect of donor age and type on outcome. Some studies have suggested more favorable outcome after transplant with young MUD as compared with older MRD. In contrast, in a large registry trial of elderly patients, Alousi et al. recently reported better survival after MRD≥50y-HSCT in comparison with MUD-HSCT, particularly for fit patients at the time of transplant.39 Compared to our study, only 38% of MRD were older than 60 years in their study. By further exploring for a donor age cut off among MRD, they observed that patients who received grafts from MRD aged ≥67 years experienced higher relapse and mortality risks compared to those transplanted from younger MRD. Which donor source is preferable between an old MRD and a younger MUD and what is the MRD age cut off, if any, above which MUD may be preferred, remain controversial and have to be investigated in further studies.
Graft CD34+and CD3+ cell doses also impacted long-term survival in our study. High CD34+ cell dose was associated with better OS and PFS and lower NRM, as previously reported.40 Faster engraftment, earlier lymphocyte recovery and lower rate of infections have been reported with higher CD34+ infused cell dose41–43 and might have contributed to our results. Some authors have, however, suggested inferior outcome after transplant with very high CD34+ cell dose (>8–10×106 cells/kg).44 We did not observe such a negative impact of very high dose (data not shown). As previously reported,45 we also observed a significant advantage of high graft CD3+ cell dose with respect to OS, PFS and NRM, even after adjustment for pre-transplant ATG use. Interestingly, patients transplanted with high graft CD34+ or CD3+ cell doses did not experience higher risks for chronic GVHD.
We failed to fully validate DRI14 for survival prediction as only patients with very high DRI (representing only 3% of patients) had significant worse OS and PFS. Our cohort differed from the training and validation cohorts originally reported by Armand et al. First, children were included in our study. Whether DRI is applicable for a pediatric population has to be evaluated. Secondly, disease distribution was different. Due to the specific recruitment of our center, there was a relative greater representation of MM and MPN (mainly myelofibrosis) in our cohort. Low relapse rate and high long-term PFS and OS were noted for patients transplanted for MPN, as previously reported.46,47 Conversely, high relapse incidence and low long-term PFS and OS were observed after PB-HSCT for MM patients, as reported by others.48,49 Thus, assignment of MPN and MM within the intermediate disease type risk category as originally suggested might have altered the stratification ability of DRI for survival in our cohort. Using an adapted index (aDRI) by reclassifying MPN and MM to the low and high disease type risk categories, respectively, we observed that aDRI better discriminated relapse and PFS than DRI. However, aDRI did not improve stratification ability for OS. Further comparisons of DRI and aDRI are needed.
As the aim of this study was to identify pre-transplant factors impacting outcome after PB-HSCT, we did not consider post-transplant events (such as acute or chronic GVHD) as covariates in our multivariate model. However, they might have influenced the results we observed.
Because HLA-matched PB-HSCT currently accounts for the majority of HSCT in adult patients with hematologic malignancies, the multivariate risk factor analysis presented here can be useful for defining clinical pre-transplant predictors of its long-term success.
Supplementary Material
Acknowledgments
The authors would like to thank Daniel J. Weisdorf, University of Minnesota Medical Center, Minneapolis, USA, for his helpful comments on the manuscript and Philippe Armand, Dana-Farber Cancer Institute, Boston, USA, for providing clarification and information about the DRI.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Karanes C, Nelson GO, Chitphakdithai P, Agura E, Ballen KK, Bolan CD, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):8–15 [DOI] [PubMed] [Google Scholar]
- 2.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367 (16):1487–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344(3):175–81 [DOI] [PubMed] [Google Scholar]
- 4.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100(5):1525–31 [DOI] [PubMed] [Google Scholar]
- 5.Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J, et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000;355(9211):1231–7 [DOI] [PubMed] [Google Scholar]
- 6.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19(16):3685–91 [DOI] [PubMed] [Google Scholar]
- 7.National Marrow Donor Program and Be The Match Foundation Web Site [Internet] Minneapolis: National Marrow Donor Program; c1996–2013. [updated 2013 May 24; cited 2013 Sep 8]. Trends in Allo Transplants; [about 4 screens]. Available from: http://marrow.org/ContentNarrow.aspx?id=2297&terms=trend+in+allogeneic/ [Google Scholar]
- 8.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110 (13):4576–83 [DOI] [PubMed] [Google Scholar]
- 9.Bacigalupo A. Matched and mismatched unrelated donor transplantation: is the outcome the same as for matched sibling donor transplantation? Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2012:223–9 [DOI] [PubMed] [Google Scholar]
- 10.Mielcarek M, Storer BE, Sandmaier BM, Sorror ML, Maloney DG, Petersdorf E, et al. Comparable outcomes after nonmyeloablative hematopoietic cell transplantation with unrelated and related donors. Biol Blood Marrow Transplant. 2007;13(12):1499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119(17):3908–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlenk RF, Dohner K, Mack S, Stoppel M, Kiraly F, Gotze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010;28(30):4642–8 [DOI] [PubMed] [Google Scholar]
- 13.Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–702 [DOI] [PubMed] [Google Scholar]
- 14.Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons: New York, 1980 [Google Scholar]
- 17.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–54 [PubMed] [Google Scholar]
- 18.Grambsch P, Therneau T. Proportional hazards tests and diagnotics based on weighted residuals. Biometrika. 1994;85:515–56 [Google Scholar]
- 19.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165 (6):710–8 [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87 [DOI] [PubMed] [Google Scholar]
- 21.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72 [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Song L. Quantifying discrimination of Framingham risk functions with different survival C statistics. Stat Med. 2012;31(15):1543–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2012;32(14):2430–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Development Core Team R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. Available from: http://www.R-project.org ISBN 3-900051-07-0 [Google Scholar]
- 27.Friedman JS, Alpdogan O, van den Brink MR, Liu C, Hurwitz D, Boyd A, et al. Increasing T-cell age reduces effector activity but preserves proliferative capacity in a murine allogeneic major histocompatibility complex-mismatched bone marrow transplant model. Biol Blood Marrow Transplant. 2004;10(7):448–60 [DOI] [PubMed] [Google Scholar]
- 28.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181(3):1835–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carreras E, Jimenez M, Gomez-Garcia V, de la Camara R, Martin C, Martinez F, et al. Donor age and degree of HLA matching have a major impact on the outcome of unrelated donor haematopoietic cell transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 2006;37(1):33–40 [DOI] [PubMed] [Google Scholar]
- 31.Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99(6):1943–51 [DOI] [PubMed] [Google Scholar]
- 32.Fabre C, Koscielny S, Mohty M, Fegueux N, Blaise D, Maillard N, et al. Younger donor’s age and upfront tandem are two independent prognostic factors for survival in multiple myeloma patients treated by tandem autologous-allogeneic stem cell transplantation: a retrospective study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Haematologica. 2012;97(4):482–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finke J, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biol Blood Marrow Transplant. 2012;18(11):1716–26 [DOI] [PubMed] [Google Scholar]
- 34.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–51 [DOI] [PubMed] [Google Scholar]
- 35.Mehta J, Gordon LI, Tallman MS, Winter JN, Evens AM, Frankfurt O, et al. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transplant. 2006;38(2):95–100 [DOI] [PubMed] [Google Scholar]
- 36.Ringden O, Labopin M, Bacigalupo A, Arcese W, Schaefer UW, Willemze R, et al. Transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical siblings in adult patients with acute myeloid leukemia and acute lymphoblastic leukemia. J Clin Oncol. 2002;20 (24):4655–64 [DOI] [PubMed] [Google Scholar]
- 37.Ayuk F, Zabelina T, Wortmann F, Alchalby H, Wolschke C, Lellek H, et al. Donor choice according to age for allo-SCT for AML in complete remission. Bone Marrow Transplant. 2013;48(8):1028–32 [DOI] [PubMed] [Google Scholar]
- 38.Kroger N, Zabelina T, de Wreede L, Berger J, Alchalby H, van Biezen A, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia. 2013;27 (3):604–9 [DOI] [PubMed] [Google Scholar]
- 39.Alousi AM, Le-Rademacher J, Saliba RM, Appelbaum FR, Artz A, Benjamin J, et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood. 2013;121(13):2567–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulsipher MA, Chitphakdithai P, Logan BR, Leitman SF, Anderlini P, Klein JP, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114(13):2606–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron F, Maris MB, Storer BE, Sandmaier BM, Panse JP, Chauncey TR, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19(5):822–8 [DOI] [PubMed] [Google Scholar]
- 42.Bittencourt H, Rocha V, Chevret S, Socie G, Esperou H, Devergie A, et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;99(8):2726–33 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura R, Auayporn N, Smith DD, Palmer J, Sun JY, Schriber J, et al. Impact of graft cell dose on transplant outcomes following unrelated donor allogeneic peripheral blood stem cell transplantation: higher CD34+ cell doses are associated with decreased relapse rates. Biol Blood Marrow Transplant. 2008;14(4):449–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohty M, Bilger K, Jourdan E, Kuentz M, Michallet M, Bourhis JH, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17(5):869–75 [DOI] [PubMed] [Google Scholar]
- 45.Cao TM, Shizuru JA, Wong RM, Sheehan K, Laport GG, Stockerl-Goldstein KE, et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005;105(6):2300–6 [DOI] [PubMed] [Google Scholar]
- 46.Deeg HJ, Gooley TA, Flowers ME, Sale GE, Slattery JT, Anasetti C, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood. 2003;102(12):3912–8 [DOI] [PubMed] [Google Scholar]
- 47.Kerbauy DM, Gooley TA, Sale GE, Flowers ME, Doney KC, Georges GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant. 2007;13(3):355–65 [DOI] [PubMed] [Google Scholar]
- 48.Gahrton G, Iacobelli S, Bandini G, Bjorkstrand B, Corradini P, Crawley C, et al. Peripheral blood or bone marrow cells in reduced-intensity or myeloablative conditioning allogeneic HLA identical sibling donor transplantation for multiple myeloma. Haematologica. 2007;92(11):1513–8 [DOI] [PubMed] [Google Scholar]
- 49.Kumar S, Zhang MJ, Li P, Dispenzieri A, Milone GA, Lonial S, et al. Trends in allogeneic stem cell transplantation for multiple myeloma: a CIBMTR analysis. Blood. 2011;118(7):1979–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.