Abstract
WW domain-containing oxidoreductase (WWOX) is highly conserved in both humans and murine. WWOX spans the second most common human chromosomal fragile site, FRA16D, and is commonly inactivated in multiple human cancers. Modeling WWOX inactivation in mice revealed a complex phenotype including postnatal lethality, defects in bone metabolism and steroidogenesis and tumor suppressor function resulting in osteosarcomas. For better understanding of WWOX roles in different tissues at distinct stages of development and in pathological conditions, Wwox conditional knockout mice were generated in which loxp sites flank exon 1 in the Wwox allele. We demonstrated that Cre-mediated recombination using EIIA-Cre, a Cre line expressed in germline, results in postnatal lethality by age of three weeks and decreased bone mineralization resembling total ablation of WWOX as in conventional null mice. This animal model will be useful to study distinct roles of WWOX in multiple tissues at different ages.
Keywords: conditional knockout, WWOX, EIIA-cre, bone
Introduction
The WW domain-containing oxidoreductase (WWOX) encodes a 46-kDa tumor suppressor protein that contains two WW domains and a short-chain dehydrogenase/reductase domain (Bednarek et al., 2000; Ried et al., 2000). Loss or reduced expression of WWOX occurs in multiple cancer types, perhaps due to its genomic location overlapping with the second most common fragile site, FRA16D, and/or hypermethylation of its promoter region in many cancer cells (Aqeilan and Croce, 2007; Del Mare et al., 2009; Paige et al., 2001) WWOX overexpression in WWOX-negative cancer cells renders these cells sensitive to apoptosis and growth arrest and reduced ability to form xenografts in immunocompromised mice (Del Mare et al., 2009). Furthermore, WWOX protein is part of a protein signaling network that suppresses oncoproteins that are implicated in the pathogenesis of cancer. This includes, for example, AP2γ (Aqeilan et al., 2004b), ErbB4 (Aqeilan et al., 2007a; Aqeilan et al., 2005), ezrin (Jin et al., 2006), c-Jun (Gaudio et al., 2006) and RUNX2 (Aqeilan et al., 2008; Kurek et al., 2010). These findings led us and others to hypothesize that WWOX acts as a tumor suppressor.
To better explore the tumor suppressor function of WWOX in vivo, we reported in 2007, the generation of total Wwox knockout (Wwox−/−) mice using conventional homologous recombination technology (Aqeilan et al., 2007c). Wwox−/− mice die prematurely at 3–4 weeks of age due to severe metabolic defect. Mice showed a significant growth retardation phenotype that was associated with defects in bone (Aqeilan et al., 2008) and steroidogenesis (Aqeilan et al., 2009). Importantly, careful analysis of Wwox−/− mice using μCT and histological sectioning detected lesions in femoral bones that resemble osteosarcoma (Aqeilan et al., 2007c; Kurek et al., 2010). Alteration of WWOX in human osteosarcoma cases and cells was later documented (Kurek et al., 2010; Yang et al., 2010). Although Wwox-heterozygous (Wwox+/−) aged mice demonstrated higher incidence of spontaneous lung and mammary tumors (Abdeen et al., 2011; Aqeilan et al., 2007c) and chemically-induced forestomach tumors (Aqeilan et al., 2007b), the fact that Wwox−/− mice die at early age precluded adult tumor analysis. Of note, Wwox-hypomorphic mice have shorter life span and display higher incidence of B-cell lymphomas (Ludes-Meyers et al., 2007) again supporting a role of WWOX as a tumor suppressor. These results prompted us to set a strategy to generate a conditional knockout mouse model for the Wwox gene in order to enable careful adult tumor analysis.
Here we report the generation of mice with conditional allele for Wwox deletion. Since a detailed phenotypic analysis was performed on conventional Wwox knockout mice, we focused in this study on the bone defect. Indeed, we found that ablation of WWOX using a Cre-Loxp strategy in germline resembles very closely that of conventional Wwox knockout model. These results suggest that this new animal model will be useful to study distinct roles of WWOX in different tissues at different ages.
Materials and Methods
Genertaion of the Wwoxflox allele
A BAC clone encompassing the Wwox locus was isolated from a mouse 129SvJ genomic library. The Wwox gene targeting construct was generated by isolating and cloning a 1.16-kb fragment containing exon 1 into Loxp-Frt-PGK-Neo-pA-Frt containing vector. A 3.05-kb fragment was then subcloned as the 5′ arm upstream of exon 1 and a 3.13-kb fragment as the 3′ arm downstream of exon 1 into the same vector. The final targeting construct contained a floxed exon 1 and Frt-PGK-Neo-pA-Frt cassette.
Electroporation of ES cells and generation of chimeras
The final plasmid was linearized using NotI, phenol-chloroform extracted, and electroporated into R1 ES cells. The selection and expansion of ES cell clones were performed according to standard methods. Genomic DNA was extracted from ~300 clones, digested with BglII, separated on a 0.8% agarose gel and hybridized with a 32P-labled probe. A 5′ external probe (187 bp) was generated by PCR using the following primers: 5′-CTTGTCTAGAGGGCTGTTTC-3′ and 5′-CCAGTCTGCAACTGAAAATAG-3′; a 3′ external probe (627 bp) was generated by PCR using the following primers: 5′-AACTCAAAGTAGAAAGAACAAGG -3′ and 5′-CTCCTTCTGCCAAATCCCGTT-3′. Southern blot screening identified eight homologous recombination clones. Three clones were injected into C57BL/6J blastocysts and implanted into foster mothers. Male chimeras derived from all 3 ES clones were selected by agouti color and mated to C57BL/6J females to identify mice with germline transmission. The EIIa-cre mice were obtained from JAX lab, ST#003314. All experiments involving mice were approved by the Hebrew University Institutional Animal Care and Use Committee.
PCR Genotyping analysis
Three primers were used: a shared 5′ primer of wild type allele recognizing Exon 1-chr8:116963753 (5′-AAGGACGGCTGGGTGTACTA-3′), a 3′primer internal to the Frt-PGK-Neo cassette (5′-ACCAAAGAACGGAGCCGGTT-3′) and a 3′ primer recognizing wild type allele chr8: 116964294 (5′-CAACCTACTAGCCTCTCCAC-3′). This assay yielded a 541 bp wild type PCR fragment and 766 bp knockout fragment. Genotyping of Cre was performed using the following primers: Forward, 5′-ATG TCC AAT TTA CTG ACC GTA CAC C-3′; 5′-Reverse, CGC CTG AAG ATA TAG AAG ATA ATC G-3′.
Skeletal preparation and histology
Radiography of dissected limbs at ages 14 days was performed after fixation in 4% paraformaldehyde at 4 °C under vacuum 2 days and rinsing in PBS using a Faxitron MX-20 specimen radiography system. Microcomputed tomography (μCT) studies were performed on limbs fixed in 70% ethanol for scanning. Qualitative and quantitative three-dimensional analysis of femurs was carried out using micro-CT imaging (μCT 40, Scanco Medical AG, Bassersdorf, Switzerland) by Stacey Russell.
RNA extraction and RT-PCR
RNA was isolated from the different tissues followed by homogenizing in TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Bones were cut at the mid-diaphysis and flushed free of marrow by PBS after removing the epiphyses from either end. The bone halves were placed and frozen in TRIzol for RNA isolation by homogenization. cDNA was synthesized with oligo(dT) primers using the Super-Script first strand synthesis kit (Invitrogen) according to the manufacturer’s protocol. Primers used were: Wwox (murine-F:5′-GGG AGC TGC TAC CAC TGT CTA-3′; murine R:5′-CCT CTC ACT GAG TTC CCA CA-3′), Ubc (F: 5′-CAGCCGTATATCTTCCCAGAC-3′; R: 5′-CTCAGAGGGATGCCAGTAATCTA- 3′).
Immunoblotting
Whole cell lysates were prepared using 0.5% NP-40-containing buffer (Aqeilan et al., 2004c). Antibodies used were polyclonal anti-WWOX (Aqeilan et al., 2004a) and monoclonal anti-GAPDH (Calbiochem).
Results
Generation of mice with targeted flexed-Wwox allele
To construct the Wwoxflox allele, we have made a targeting vector containing two Loxp sites flanking exon 1. In this way, existence of Cre recombinase would lead to deletion of exon 1 and subsequent inactivation of Wwox. To do so, we inserted a 2.8-kb middle fragment containing Loxp sites flanking exon 1 and an FRT-PGK-NEOR-pA-FRT sequence into the targeting vector, then ligated a 3.05-kb fragment as the 5′ homology recombination arm. A 3.13-kb fragment containing most of intron 1 to form the 3′ homology recombination arm was then inserted into the targeting vector (Fig. 1a). The Not I-linearized targeting vector Wwoxflox was transfected into R1 embryonic stem (ES) cells by electroporation. Eight targeted ES clones were detected by Southern blot using a 5′ flanking external probe (Fig. 1b). Three positive ES clones were microinjected into blastocycts harvested from C57/BL6 mice according to standard protocols. Proper recombination of targeting vector in mice was assessed by Southern blot (data not shown).
Fig. 1.
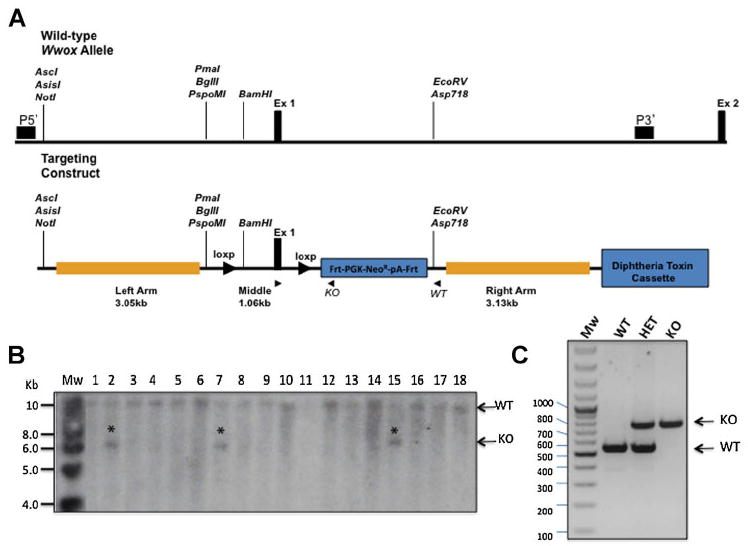
Conditional targeted disruption of the Wwox gene. A: The Wwox genomic locus was altered by inserting two loxp sites flanking Exon 1 and thus generating floxed-Wwox allele. A targeting cassette containing chimeric sequences of Frt-PGK-NeoR-pA-Frt was inserted 5′ of Exon 1. A 5′ or 3′ genomic probe (horizontal dark box) that recognized a 10 kb WT fragment and a 6 kb targeted BglII fragment was used for genotyping by Southern blotting. Three primers (arrows), a shared 5′ primer (E1), distinct 3′ primers that recognized wild type locus (WT), and the other 3′ primers specific for targeting cassette (KO) allowed PCR genotyping. B: Southern blot screening analysis of genomic DNA extracted from mouse targeted ES cells and digested with BglII showing 3 positive clones (*). (C) PCR genotype of gDNA extracted from mouse-tails showing the different genotypes. Mw, molecular weight; WT, wildtype; HET, heterozugous; KO, knockout.
We generated Wwoxflox/flox mice by intercrossing of Wwoxflox/+ heterozygous mice. PCR was used to identify Wwoxflox/flox (Fig. 1c) [WT allele 541bp, KO allele 766]. Further, we utilized quantitative RT-PCR analysis to examine Wwox mRNA as well as Western blot to determine WWOX protein expression. We observed intact WWOX expression in most mice although some founders of Wwoxflox/flox mice display reduced Wwox levels (Fig. 2a, b), likely due to the presence of a regulatory element in exon 1. Nevertheless, phenotypic analysis of these mice for ~2 years did not reveal any effect on life span or physiology different than their control wild-type littermates (data not shown).
Fig. 2.
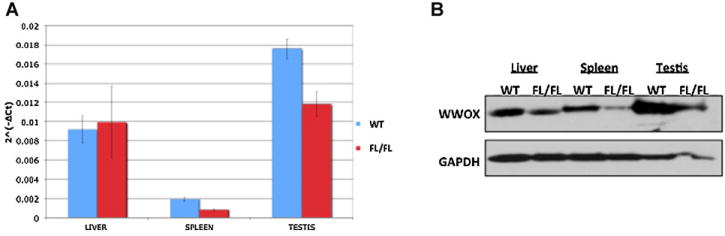
Validation of WWOX expression in Wwoxfl/fl mice. A: Real-time PCR of mouse tissues (liver, spleen, and testis) indicating expression of Wwox mRNA in homozygous Wwoxfl/fl mice. B: Western blot analysis of mouse tissues (liver, spleen, and testis) with anti-WWOX antibody showing presence of WWOX. GAPDH levels were used for normalization.
Cre-Recombinase Deletion of the Wwox Gene ablates WWOX expression
To test whether exon 1 in Wwoxflox allele can be deleted by the Cre recombinase, Wwoxflox/flox mice were crossed with the EIIa-Cre transgenic mice that express Cre recombinase in germline (Newton et al., 2008). The offspring were identified by PCR genotyping as in Fig. 1c and Cre general primers. A Mendelian ratio of the three expected alleles (Wwoxflox/flox, Wwoxflox/Δ, WwoxΔ/Δ) was obtained (data not shown). Expression of Wwox mRNA and WWOX protein was further validated by real-time PCR and Western blot in different tissues, respectively. As shown in Fig. 3, WWOX expression is significantly depleted in major tissues including liver, lung and skeletal muscle. Immunohistochemical analyses of WWOX expression also validated WWOX deletion in bone tissues (Fig. 3c), a major known tissue affected by WWOX ablation (Aqeilan et al., 2008). As shown in Fig. 3c, the highest expression of WWOX in Wwoxflox/flox control mice is at the bone forming front under the growth plate.
Fig. 3.
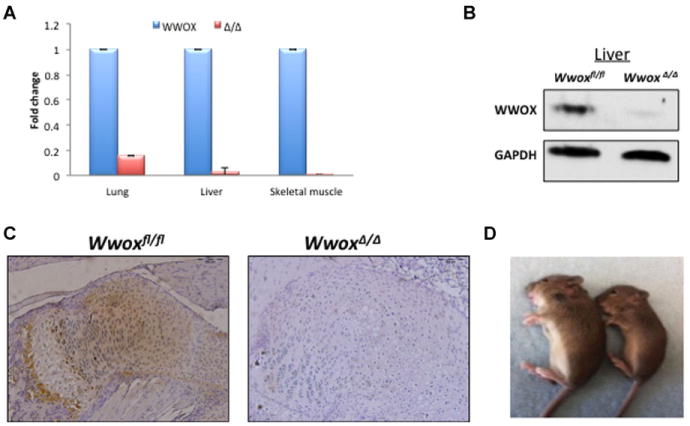
Total Wwox ablation using EIIA-cre transgenic mice. Deletion of Wwoxfl/fl alleles was validated using a general deleter mouse strain carrying the Cre-recombinase gene under control of the adenoviral EIIA-promoter. A: Real-time PCR of mouse tissues isolated from control Wwoxfl/fl (WWOX) and Wwoxfl/fl –EIIA-cre (WwoxΔ/Δ) mice. Ubc levels was used for normalization. B: Western blot analysis of liver tissues using anti-WWOX antibody showing deletion of WWOX in WwoxΔ/Δ mice. GAPDH levels were used for normalization. C: Immunohistochemical staining of bone tissues using anti-WWOX antibody showing absent WWOX expression (brown) in WwoxΔ/Δ mice. D: A photograph showing growth retardation of WwoxΔ/Δ mice (right) as compared to Wwoxfl/fl control mice (left) at age 18 days.
Phenotypic analysis of WwoxΔ/Δ mice
To assess phenotypic abnormalities of WwoxΔ/Δ mice, we compared them with those that were reported previously in conventional Wwox−/− mice (Aqeilan et al., 2008; Aqeilan et al., 2007c). Conventional Wwox−/− mice displayed growth retardation and postnatal lethality due to a severe metabolic disorder mainly characterized by hypoglycemia (Aqeilan et al., 2008). Similarly, we observed that WwoxΔ/Δ mice display significant growth retardation (Fig. 3d) and die by three weeks of age also due to severe hypoglycemia (data not shown).
Another major phenotype in our previously reported conventional Wwox knockout mice includes defects in bone formation; these mice display reduced bone mineralization (Aqeilan et al., 2008). To further validate whether ablation of WWOX using our Cre-loxP strategy harbors a similar bone phenotype, femoral bones of WwoxΔ/Δ and control mice were examined by X-ray and micro-computed tomography (μCT). Our analysis revealed that femurs of WwoxΔ/Δ mice indeed display an osteopenic phenotype (Fig. 4).
Fig. 4.
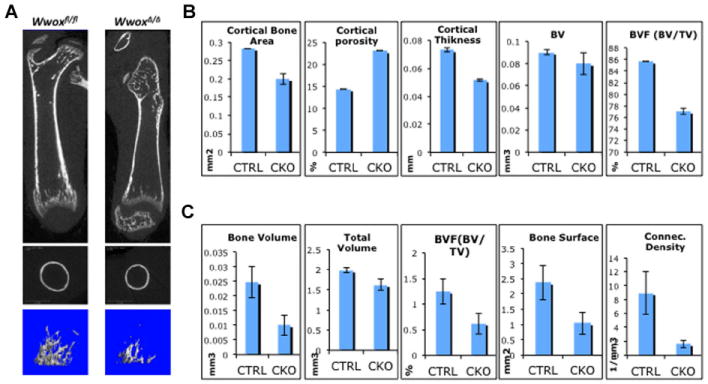
Delayed bone formation in WwoxΔ/Δ mice. A: Microcomputed tomography (μCT) three-dimensional images of control (Wwoxfl/fl) and knockout (WwoxΔ/Δ) at 14 days old mice. Top parts, representative image from the midsection of femur that shows less trabecular bone and a delay in the secondary center of ossification in WwoxΔ/Δ mice; middle parts, cross-section at mid-diaphysis shows thin cortical bone of WwoxΔ/Δ; Lower parts, μCT image of the metaphysis region of Wwoxfl/fl and WwoxΔ/Δ femur at Day 14 showing less volume of trabecular bone. B, C: Quantitation of selected cortical (B) or trabecular (C) bone formation parameters from μCT data analyses as indicated. Parameters of control Wwoxfl/fl mice (CTRL) are compared to those of WwoxΔ/Δ (CKO). P value is at least <0.05.
Limbs of the WwoxΔ/Δ mice revealed not only a size difference proportional to the animal weight, but radiography revealed that KO limbs exhibited a significant decreased density of trabeculae bone and a thinner cortex compared with WT mice as also shown in Fig. 4a. The three-dimensional μCT images of femur metaphysis in mice at day 14 showed reduced cortical and trabecular bone parameters in WwoxΔ/Δ mice (Fig. 4a–c). The most significant difference observed in WwoxΔ/Δ mice was in cortical bone thickness and area as well as trabecular bone volume (p <0.001) (Fig. 4b, c). The quality of bone was severely compromised by the increased porosity of cortical bone and decreased in connectivity density in trabecular bone.
Consistently, histologic sectioning of limbs revealed fewer trabeculae in WwoxΔ/Δ mice compared with WT (Fig. 5a). Careful histological analysis of the cells forming bone tissue around trabeculae in WwoxΔ/Δ mice femurs revealed the presence of malignant appearing cells in bone marrow of these mice (Fig. 5b). Pockets of cells on the bone surface were characterized by increased nuclei, a marker of tumor cells, but were very few in number. Given the complex phenotype of these mice and their death before weaning age, it will be necessary to examine the specific effect of WWOX ablation in distinct bone cell populations in future studies.
Fig. 5.
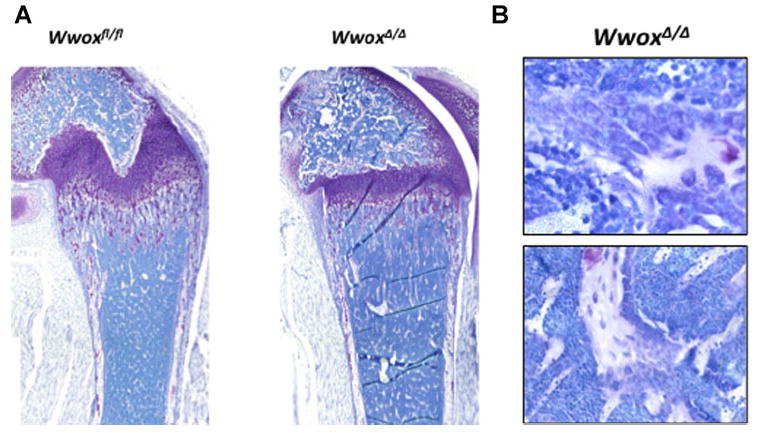
Histologic detection of tumor-like cells in in WwoxΔ/Δ femorla bones. A: Toluidine blue stained sections of demineralized femoral bones of 14-day-old mice showing less trabceulae in WwoxΔ/Δ as compared to control mice. Growth plate and cartilage tissue within trabecular bone spicules are identified by purple color. B: Histologic sectioning of femoral bone from 14 days old WwoxΔ/Δ mouse showing malignant osteoblasts.
Discussion
The WWOX tumor suppressor is commonly deleted or altered in most human cancers (Del Mare et al., 2011a; Del Mare et al., 2009; Salah et al., 2010). Many studies have shed light on the fact that WWOX genomic or epigenetic alteration is associated with tumor aggressiveness and/or patient survival. Therefore, there have been several attempts of our lab and others to study WWOX loss in biology and pathology (Aqeilan and Croce, 2007; Del Mare et al., 2011b). Animal models have been instrumental in the study of genes involved in human cancer. Our studies were first to demonstrate targeted ablation of Wwox in mice and the complex phenotype associated with it (Aqeilan et al., 2008; Aqeilan et al., 2007c). This was followed by work of the Ludes-Meyers et al. who generated a hypomorphic mouse model of Wwox in which shorter life span was shown (Ludes-Meyers et al., 2007). Recently, mice carrying a conditional allele of Wwox, similar to our mice here, were reported (Ludes-Meyers et al., 2009). Characterization of these mice confirmed our initial observations of Wwox conventional knockout mice including, postnatal lethality due to severe metabolic phenotype, growth retardation and bone growth defects. The present study here also reports the generation and characterization of conditional knockout mice of the Wwox allele and confirms that WWOX ablation leads to the deregulation of osteoprogenitor cells in the bone marrow that transform into osteosarcoma like cells. Of note, there have also been some differences and inconsistences in analyzing these mouse models related to steroidogenesis defect and tumors resembling pre-osteosarcomas. We believe that future detailed analyses of WWOX loss in specific tissues will contribute to a better understanding of the role of WWOX in biology and tumorigenesis.
In conclusion, we have generated a mouse model with a conditional allele at the Wwox locus (Wwoxfl/fl). Our analysis demonstrated that homozygous Cre-recombinase mediated deletion of Wwox Exon 1 lead to complete ablation of WWOX expression. Loss of WWOX expression by this approach resulted in early lethality of the mouse that informs a phenotype similar to previously reported studies on Wwox targeted deletion. Wwoxfl/fl mice represent an important tool for studying WWOX function in normal mouse physiology, such as studying Wwox specific ablation in mammary gland development (Abdeen et al., 2012), and adult tissue specific tumorigenesis.
Acknowledgments
Authors are grateful to Dr. Eugenio Gaudio, Maureen Mork and Christopher Lemmon for technical help with ES screening. This work was supported by the Israeli Science Foundation grant (ISF #08-1331), EU-FP7 Marie Curie Re-integration grant, Bi-national Science Foundation (BSF #2011330) and NIH grants: PO1 CA13922, R37 DE012528, and RO1 AR039588 to (G.S. Stein, J.L. Stein and J.B. Lian).
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.24308]
Competing interests statement.
Authors declare no competing financial interests.
References
- Abdeen SK, Salah Z, Khawaled S, Aqeilan RI. Characterization of WWOX inactivation in murine mammary gland development. J Cell Physiol. 2012 doi: 10.1002/jcp.24310. In press. [DOI] [PubMed] [Google Scholar]
- Abdeen SK, Salah Z, Maly B, Smith Y, Tufail R, Abu-Odeh M, Zanesi N, Croce CM, Nawaz Z, Aqeilan RI. Wwox inactivation enhances mammary tumorigenesis. Oncogene. 2011;30(36):3900–3906. doi: 10.1038/onc.2011.115. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Croce CM. WWOX in biological control and tumorigenesis. J Cell Physiol. 2007;212(2):307–310. doi: 10.1002/jcp.21099. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H, Croce CM, Elenius K. Association of Wwox with ErbB4 in Breast Cancer. Cancer Res. 2007a;67(19):9330–9336. doi: 10.1158/0008-5472.CAN-07-2147. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005;65(15):6764–6772. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM. Inactivation of the Wwox Gene Accelerates Forestomach Tumor Progression In vivo. Cancer Res. 2007b;67(12):5606–5610. doi: 10.1158/0008-5472.CAN-07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Hagan JP, de Bruin A, Rawahneh M, Salah Z, Gaudio E, Siddiqui H, Volinia S, Alder H, Lian JB, Stein GS, Croce CM. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology. 2009;150(3):1530–1535. doi: 10.1210/en.2008-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Hassan MQ, de Bruin A, Hagan JP, Volinia S, Palumbo T, Hussain S, Lee SH, Gaur T, Stein GS, Lian JB, Croce CM. The WWOX tumor suppressor is essential for post-natal survival and normal bone metabolism. J Biol Chem. 2008;283(31):21629–21639. doi: 10.1074/jbc.M800855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, Baffa R, Huebner K, Edmonds P, Croce CM. Loss of WWOX expression in gastric carcinoma. Clin Cancer Res. 2004a;10(9):3053–3058. doi: 10.1158/1078-0432.ccr-03-0594. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res. 2004b;64(22):8256–8261. doi: 10.1158/0008-5472.CAN-04-2055. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci U S A. 2004c;101(13):4401–4406. doi: 10.1073/pnas.0400805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, Lian JB, Croce CM. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci U S A. 2007c;104(10):3949–3954. doi: 10.1073/pnas.0609783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60(8):2140–2145. [PubMed] [Google Scholar]
- Del Mare S, Kurek KC, Stein GS, Lian JB, Aqeilan RI. Role of the WWOX tumor suppressor gene in bone homeostasis and the pathogenesis of osteosarcoma. Am J Cancer Res. 2011a;1(5):585–594. [PMC free article] [PubMed] [Google Scholar]
- Del Mare S, Kurek KC, Stein GS, Lian JB, Aqeilan RI. Role of the WWOX tumor suppressor gene in bone homeostasis and the pathogenesis of osteosarcoma. Am J Cancer Res. 2011b;1(5):585–594. [PMC free article] [PubMed] [Google Scholar]
- Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem. 2009;108(4):737–745. doi: 10.1002/jcb.22298. [DOI] [PubMed] [Google Scholar]
- Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res. 2006;66(24):11585–11589. doi: 10.1158/0008-5472.CAN-06-3376. [DOI] [PubMed] [Google Scholar]
- Jin C, Ge L, Ding X, Chen Y, Zhu H, Ward T, Wu F, Cao X, Wang Q, Yao X. PKA-mediated protein phosphorylation regulates ezrin-WWOX interaction. Biochem Biophys Res Commun. 2006;341(3):784–791. doi: 10.1016/j.bbrc.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, Amir G, Gebhardt M, Warman M, Stein GS, Stein JL, Lian JB, Aqeilan RI. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res. 2010;70(13):5577–5586. doi: 10.1158/0008-5472.CAN-09-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Nunez MI, Conti CJ, Parker-Thornburg J, Bedford MT, Aldaz CM. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer. 2007;46(12):1129–1136. doi: 10.1002/gcc.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One. 2009;4(11):e7775. doi: 10.1371/journal.pone.0007775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, Komuves L, French DM, Ferrando RE, Lam C, Compaan D, Yu C, Bosanac I, Hymowitz SG, Kelley RF, Dixit VM. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134(4):668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci U S A. 2001;98(20):11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet. 2000;9(11):1651–1663. doi: 10.1093/hmg/9.11.1651. [DOI] [PubMed] [Google Scholar]
- Salah Z, Aqeilan R, Huebner K. WWOX gene and gene product: tumor suppression through specific protein interactions. Future Oncol. 2010;6(2):249–259. doi: 10.2217/fon.09.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cogdell D, Yang D, Hu L, Li H, Zheng H, Du X, Pang Y, Trent J, Chen K, Zhang W. Deletion of the WWOX gene and frequent loss of its protein expression in human osteosarcoma. Cancer Lett. 2010;291(1):31–38. doi: 10.1016/j.canlet.2009.09.018. [DOI] [PubMed] [Google Scholar]


